All Photos(1)
About This Item
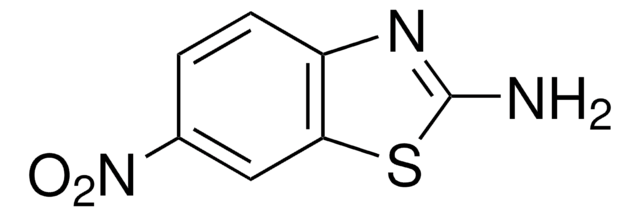
Empirical Formula (Hill Notation):
C7H4N2O2S
CAS Number:
Molecular Weight:
180.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
175-178 °C (lit.)
functional group
nitro
SMILES string
[O-][N+](=O)c1ccc2ncsc2c1
InChI
1S/C7H4N2O2S/c10-9(11)5-1-2-6-7(3-5)12-4-8-6/h1-4H
InChI key
QLUFBCVWKTWKBF-UHFFFAOYSA-N
General description
The XCORFE (Xnucleus-proton correlation with fixed evolution time) pulse sequence of 6-nitrobenzothiazole has been tested.
Application
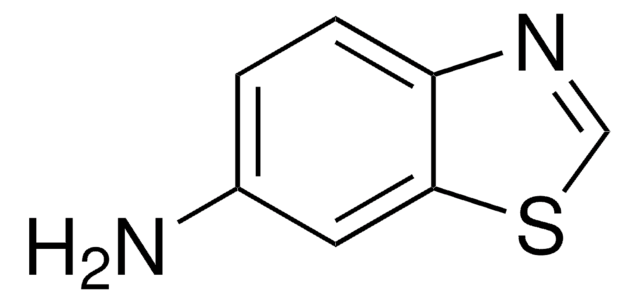
6-Nitrobenzothiazole may be used in the preparation of 4-amino-3-sulfanylbenzonitrile and 2-amino-5-nitrobenzothiole, via alkaline hydrolysis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Heterocyclic Amplifiers of Phleomycin. IX. Some Derivatives of Fused and Unfused Mono-and Di-aza Heterocycles.
Barlin GB and Ireland SJ.
Australian Journal of Chemistry, 38(11), 1685-1691 (1985)
Assignment of quaternary carbons in aromatic compounds by long-range heteronuclear shift correlated 2D-NMR spectroscopy and its application to acteoside.
Numata A, et al.
Agricultural and Biological Chemistry, 51(4), 1199-1201 (1987)
Livio Racané et al.
Bioorganic & medicinal chemistry, 18(3), 1038-1044 (2010-01-12)
The efficient synthesis of new bis-substituted nitro-amidino, amino-amidino (10a, 10b-13a, 13b) and previously prepared diamidino 2-phenyl-benzothiazoles (9a, 9b) is described. The compounds 11a and 11b were prepared by recently developed methodology of the key precursors in zwitterionic form 8a and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service