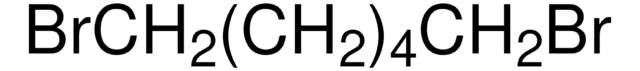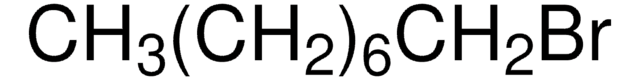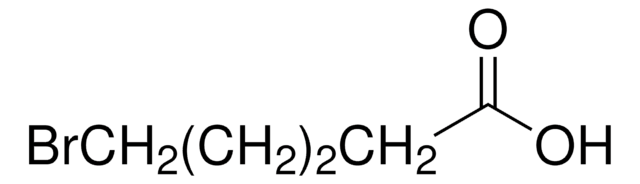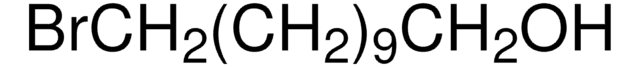467952
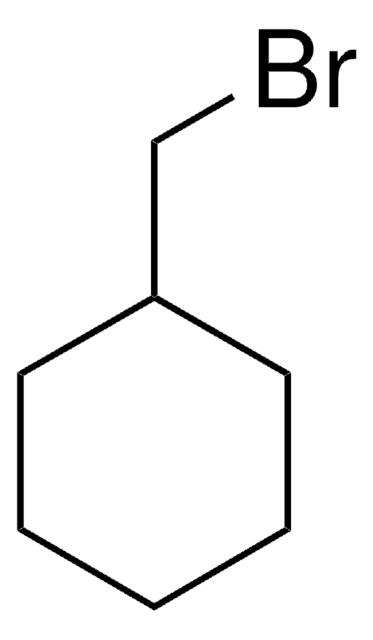
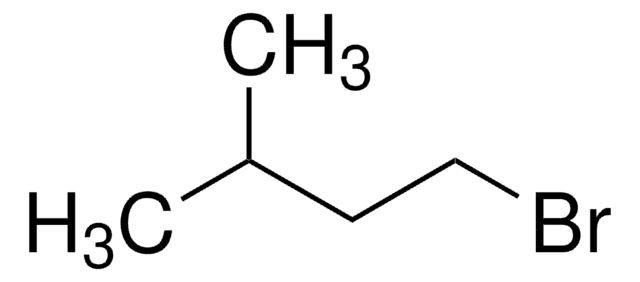
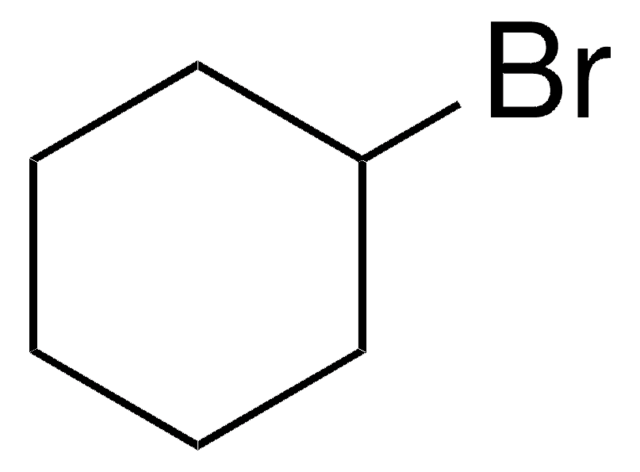
1-Bromo-2-cyclohexylethane
98%
Synonym(s):
2-Cyclohexylethyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
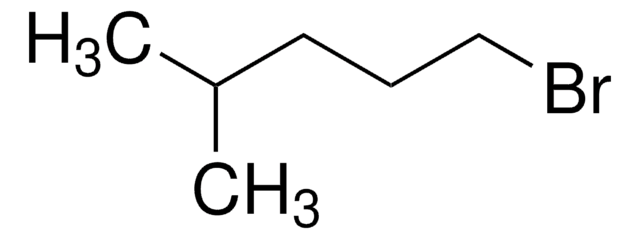
C6H11CH2CH2Br
CAS Number:
Molecular Weight:
191.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay:
98%
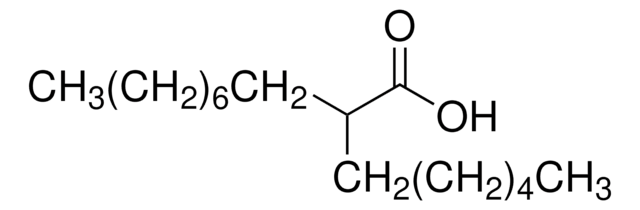
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.49 (lit.)
bp
209 °C (lit.)
density
1.221 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
BrCCC1CCCCC1
InChI
1S/C8H15Br/c9-7-6-8-4-2-1-3-5-8/h8H,1-7H2
InChI key
JRQAAYVLPPGEHT-UHFFFAOYSA-N
General description
1-Bromo-2-cyclohexylethane is a halogenated hydrocarbon. Reaction of 1-bromo-2-cyclohexylethane with pillar[5]arene has been investigated.
Application
1-Bromo-2-cyclohexylethane may be used in the synthesis of 2-cyclohexylethyloxy-4-nitroaniline.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Peter J Cragg et al.
Chemical Society reviews, 41(2), 597-607 (2011-08-02)
Pillar[5]arenes are [1(5)]paracyclophane derivatives consisting of 1,4-disubstituted hydroquinones linked by methylene bridges in the 2,5-positions. The first report of these novel macrocycles was in 2008, when 1,4-dimethoxypillar[5]arene was prepared in 22% yield, and subsequent improvements in synthetic methods have allowed
Min Wang et al.
Bioorganic & medicinal chemistry letters, 17(2), 332-336 (2006-11-11)
Aromatase is a particularly good target in the treatment of estrogen receptor positive breast cancer. Novel carbon-11 labeled sulfonanilide analogues, N-[11C]methyl-N-(2-alkyloxy-4-nitrophenyl)-methanesulfonamides ([11C]3a-f, alkyl=propyl, isopropyl, 1-ethyl-propyl, cyclopentyl, cyclohexyl, and cyclohexylethyl), were designed and synthesized as potential PET agents for imaging of
Isomerization of alkyl branched alkynoic acids.
Abrams S R.
Canadian Journal of Chemistry, 64(3), 457-463 (1986)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service