459135
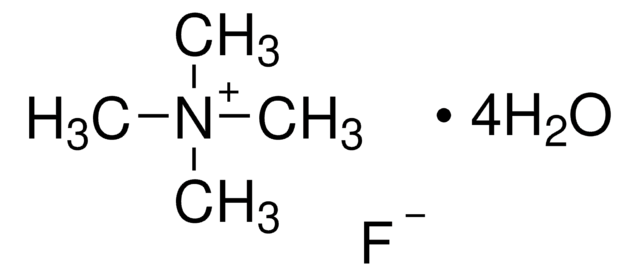
Tetramethylammonium fluoride
97%
Synonym(s):
TMAF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)4NF
CAS Number:
Molecular Weight:
93.14
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
170 °C (dec.) (lit.)
functional group
amine
SMILES string
[F-].C[N+](C)(C)C
InChI
1S/C4H12N.FH/c1-5(2,3)4;/h1-4H3;1H/q+1;/p-1
InChI key
GTDKXDWWMOMSFL-UHFFFAOYSA-M
Application
Reactant for:
- Halide-induced ring opening of 2-substituted aziridinium salts
- Synthesis of fluoro aromatics via fluorodenitration reactions
- Synthesis of sevoflurane in ionic liquids by halogen-exchange fluorination
- Synthesis of fluorinated Poly(oxomolybdates)
Tetramethylammonium fluoride may be used in combination with sulfuryl fluoride for the conversion of aryl phenols to aryl fluorides. It can react with aminosilanes to generate onium amide bases in situ, which can deprotonate heteroarenes.
Useful for halogen exchange.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nucleophilic deoxyfluorination of phenols via aryl Fluorosulfonate intermediates.
Schimler SD, et al.
Journal of the American Chemical Society, 139(4), 1452-1455 (2017)
Use of tetramethylammonium fluoride (TMAF) and alkali metal alkoxides as an activator for catalytic deprotonative functionalization of heteroaromatic C (sp2)?H bonds.
Inamoto K, et al.
Tetrahedron, 70(43), 7917-7922 (2014)
Martin-Louis Y Riu et al.
Science advances, 6(13), eaaz3168-eaaz3168 (2020-04-02)
This exploratory synthesis investigation was undertaken to determine the viability of replacing a single carbon vertex with another p-block element in a highly strained tetrahedrane molecule. Phosphorus was selected for this purpose because the stable molecular form of elemental phosphorus
Hui Chong et al.
Langmuir : the ACS journal of surfaces and colloids, 28(4), 2091-2098 (2011-11-08)
A new water-soluble conjugated polymer containing fluorene and boron-dipyrromethene repeat units in the backbones (PBF) that exhibits red emission was synthesized and characterized. Cationic PBF forms uniform nanoparticles with negatively charged disodium salt 3,3'-dithiodipropionic acid (SDPA) in aqueous solution through
Marta Matvienko et al.
PloS one, 8(2), e55913-e55913 (2013-02-15)
Several applications of high throughput genome and transcriptome sequencing would benefit from a reduction of the high-copy-number sequences in the libraries being sequenced and analyzed, particularly when applied to species with large genomes. We adapted and analyzed the consequences of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service