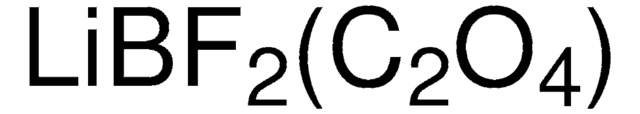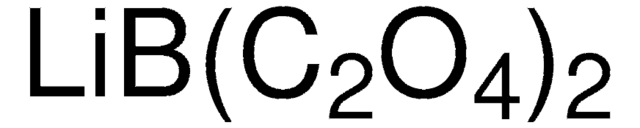450227
Lithium hexafluorophosphate
battery grade, ≥99.99% trace metals basis
Synonym(s):
Lithium phosphorus fluoride
About This Item
Recommended Products
grade
battery grade
Assay
≥99.99% trace metals basis
form
powder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤100.0 ppm Trace Metal Analysis
mp
200 °C (dec.) (lit.)
application(s)
battery manufacturing
greener alternative category
SMILES string
[Li+].F[P-](F)(F)(F)(F)F
InChI
1S/F6P.Li/c1-7(2,3,4,5)6;/q-1;+1
InChI key
AXPLOJNSKRXQPA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other Notes
Preparation and characterization of lithium hexafluorophosphate for lithium-ion battery electrolyte.
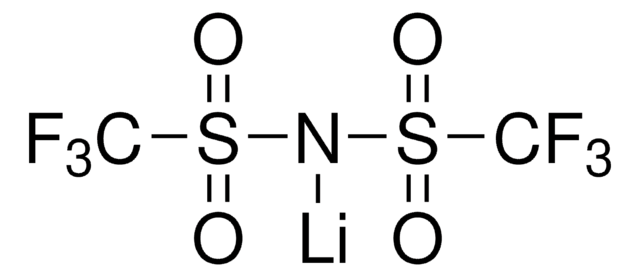
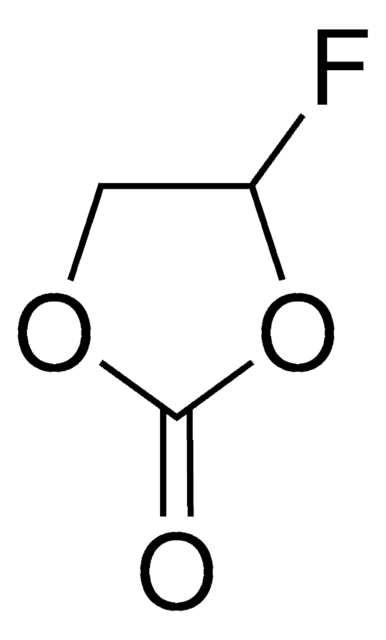
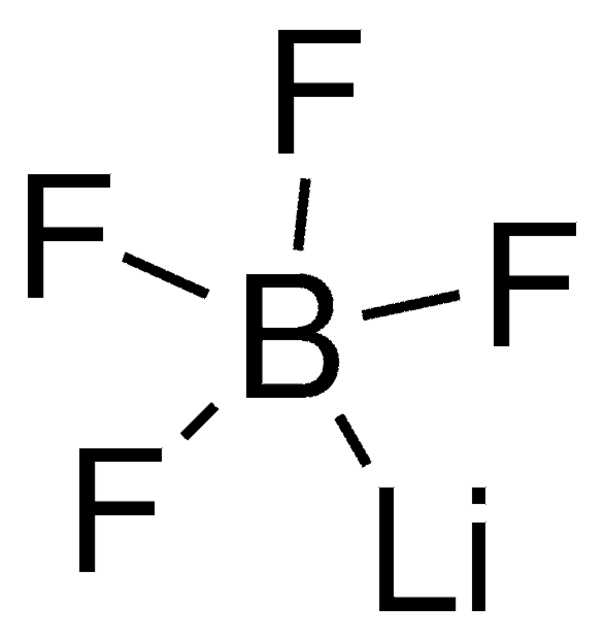
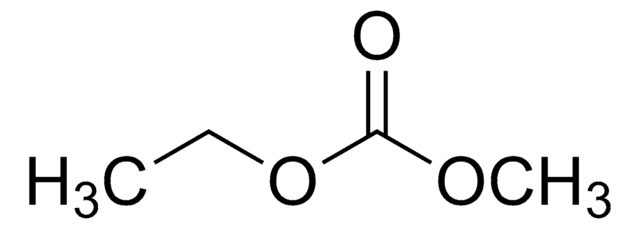
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Skin Corr. 1A - STOT RE 1 Inhalation
Target Organs
Bone,Teeth
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Increasing fuel costs and concerns about greenhouse gas emissions have spurred the growth in sales of hybrid electric vehicles (HEVs) that carry a battery pack to supplement the performance of the internal combustion engine (ICE).
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
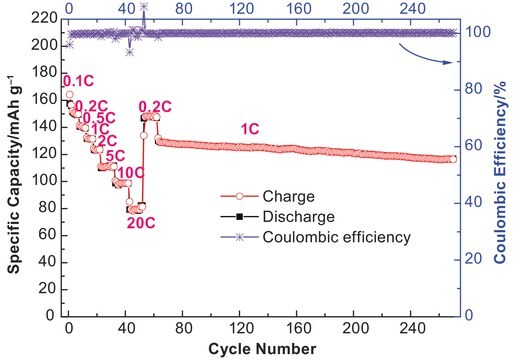
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
Related Content
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service