All Photos(1)
About This Item
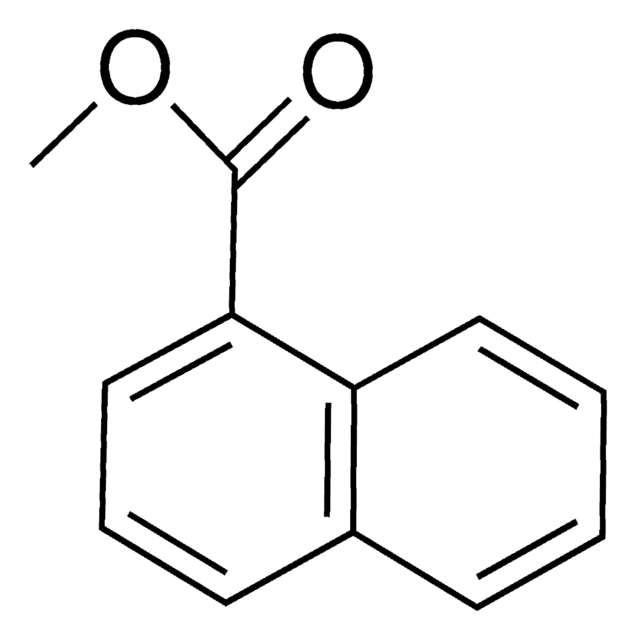
Linear Formula:
C10H7CO2CH3
CAS Number:
Molecular Weight:
186.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
290 °C (lit.)
mp
75-77 °C (lit.)
SMILES string
COC(=O)c1ccc2ccccc2c1
InChI
1S/C12H10O2/c1-14-12(13)11-7-6-9-4-2-3-5-10(9)8-11/h2-8H,1H3
InChI key
IODOXLXFXNATGI-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cucurbit [8] uril-mediated photodimerization of alkyl 2-naphthoate in aqueous solution.
Lei L, et al.
Tetrahedron Letters, 49(9), 1502-1505 (2008)
Molecular association of singlet excited state methyl 2-naphthoate; effects of excimer formation on photocycloaddition.
Chow YL and Johansson CI.
Research on Chemical Intermediates, 19(3), 191-209 (1993)
Stereochemistry of a cubane-like photodimer of methyl 2-naphthoate.
Lei L, et al.
Tetrahedron Letters, 47(27), 4725-4727 (2006)
Photochemical synthesis. VI. The formation of benzosemibullvalene derivatives in the photoaddition of diphenylacetylene to methyl 2-naphthoate, a degenerate thermal isomerization of the benzosemibullvalene skeleton.
Sugowdz G, et al.
Australian Journal of Chemistry, 26(1), 147-171 null
Rafał Frański et al.
Journal of mass spectrometry : JMS, 53(5), 379-384 (2018-02-15)
Gas phase decompositions of protonated methyl benzoate and its conjugates have been studied by using electrospray ionization-collision induced dissociation-tandem mass spectrometry. Loss of CO2 molecule, thus transfer of methyl group, has been observed. In order to better understand this process
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service