421804
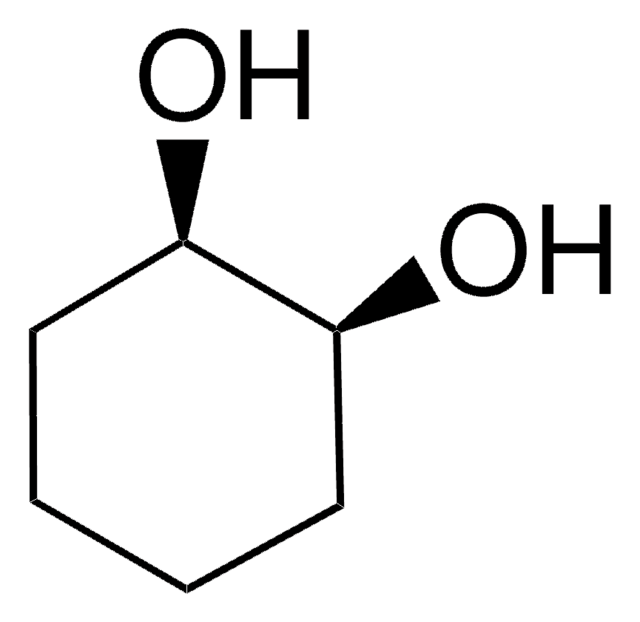
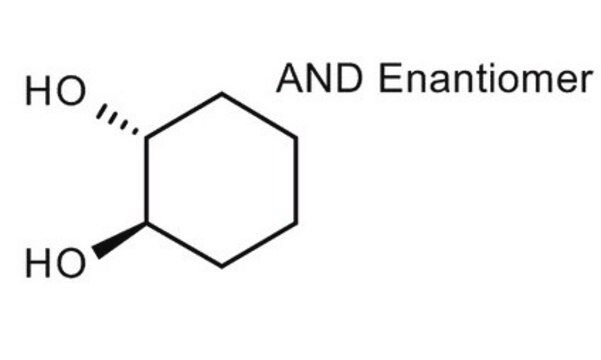
(1S,2S)-trans-1,2-Cyclohexanediol
99%
Synonym(s):
(1S)-trans-1,2-Cyclohexanediol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein:
1901343
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]20/D +39°, c = 1.6 in H2O
optical purity
ee: 99% (GLC)
mp
107-109 °C (lit.)
functional group
hydroxyl
SMILES string
O[C@H]1CCCC[C@@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6-/m0/s1
InChI key
PFURGBBHAOXLIO-WDSKDSINSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(1S,2S)-trans-1,2-Cyclohexanediol may be used in the preparation of (1S,2S)-1,2-cyclohexanediyl bis(4-vinylbenzoate) by reacting with 4-vinylbenzoyl chloride. It may also be used as a chiral ligand for the preparation of non-racemic hydroxy phosphonates via titanium alkoxide-catalyzed addition of dimethyl phosphite to the corresponding aldehydes.
C2-symmetric chiral diol with versatile applications as a chiral auxiliary, building block, and chiral ligand.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The synthesis of 1-hydroxy phosphonates of high enantiomeric excess using sequential asymmetric reactions: titanium alkoxide-catalyzed P C bond formation and kinetic resolution.
Rowe BJ and Spilling CD.
Tetrahedron Asymmetry, 12(12), 1701-1708 (2001)
Chirality induction in cyclocopolymerization. 14. Template effect of 1, 2-cycloalkanediol in the cyclocopolymerization of bis (4-vinylbenzoate) s with styrene.
Kakuchi T, et al.
Macromolecules, 34(1), 38-43 (2001)
Marshall, J.A. Xie, S.
The Journal of Organic Chemistry, 60, 7230-7230 (1995)
Stolle, A. et al.
Tetrahedron Letters, 35, 3521-3521 (1994)
Pichon, M. Figadere, B.
Tetrahedron Asymmetry, 7, 927-927 (1996)
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service