340529
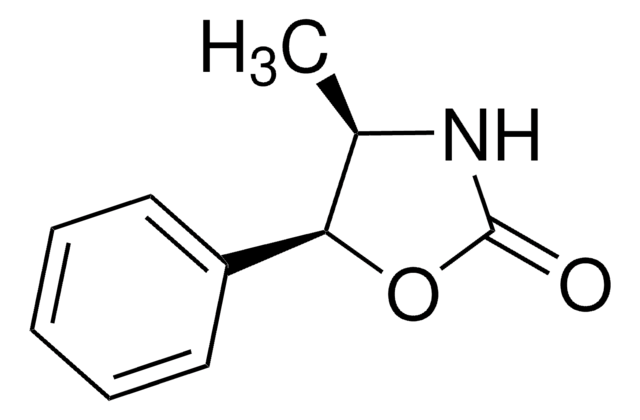
(4S,5R)-(−)-4-Methyl-5-phenyl-2-oxazolidinone
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H11NO2
CAS Number:
Molecular Weight:
177.20
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D −168°, c = 2 in chloroform
optical purity
ee: 99% (HPLC)
mp
121-123 °C (lit.)
functional group
phenyl
SMILES string
C[C@@H]1NC(=O)O[C@@H]1c2ccccc2
InChI
1S/C10H11NO2/c1-7-9(13-10(12)11-7)8-5-3-2-4-6-8/h2-7,9H,1H3,(H,11,12)/t7-,9-/m0/s1
InChI key
PPIBJOQGAJBQDF-CBAPKCEASA-N
Application
(4S,5R)-(-)-4-Methyl-5-phenyl-2-oxazolidinone may be used to synthesize (4S,5R)-N-tert-butyloxycarbonyl)-4-methyl-5-carboxy-2-oxazolidinone and (+)-pumiliotoxin B.
Versatile chiral auxiliary for asymmetric synthesis. For a review, see Aldrichimica Acta.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Antibody catalysis of peptidyl-prolyl cis-trans isomerization in the folding of RNase T1.
Ma L, et al.
Proceedings of the National Academy of Sciences of the USA, 95(13), 7251-7256 (1998)
Efficient Total Syntheses of Pumiliotoxins A and B. Applications of Iodide-Promoted Iminium Ion- Alkyne Cyclizations in Alkaloid Construction.
Lin N-H, et al.
Journal of the American Chemical Society, 118(38), 9062-9072 (1996)
Ager, D.J., et al.
Aldrichimica Acta, 30, 3-3 (1997)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service