328650
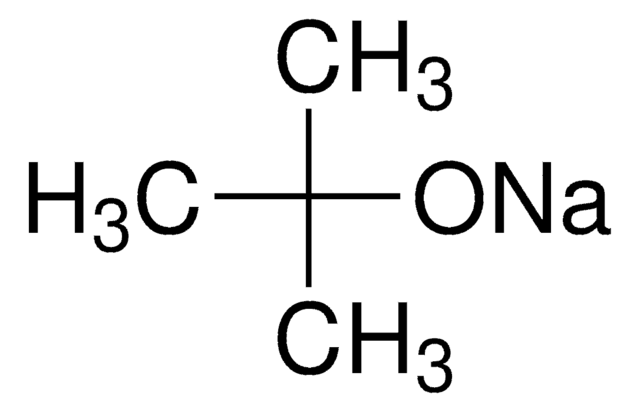
Potassium tert-butoxide solution
1.0 M in THF
Synonym(s):
Potassium tert-butylate, Potassium-2-methylpropan-2-olate solution
About This Item
Recommended Products
form
liquid
Quality Level
reaction suitability
core: potassium
concentration
1.0 M in THF
density
0.902 g/mL at 25 °C
SMILES string
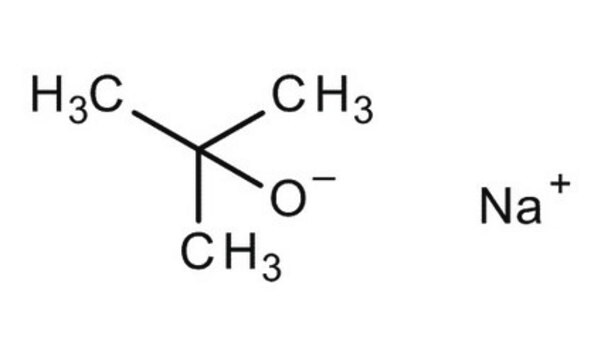
[K+].CC(C)(C)[O-]
InChI
1S/C4H9O.K/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
LPNYRYFBWFDTMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a catalyst for the interesterification of rapeseed oil with methyl acetate.
- To promote Sommelet–Hauser rearrangements of N-benzylic amino acid-derived ammonium ylides under mild conditions.
- As a metal-organic precursor in the synthesis of KNbO3 ferroelectric films by the chemical vapor deposition method.
- To prepare 3-potassiooxamethylpyridine catalyst.
- As a strong alkoxide base reagent.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
-2.2 °F - closed cup
Flash Point(C)
-19 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service