All Photos(1)
About This Item
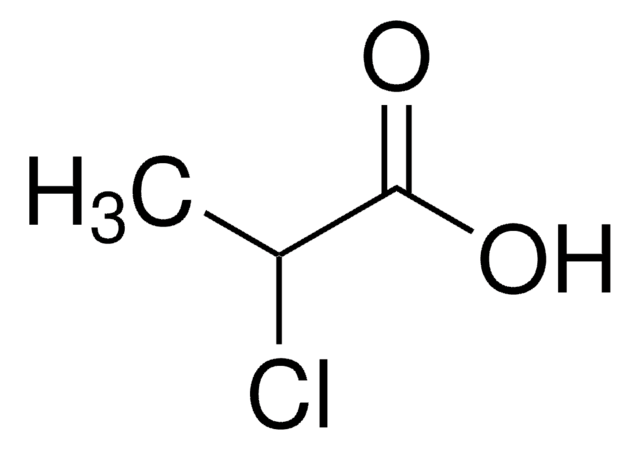
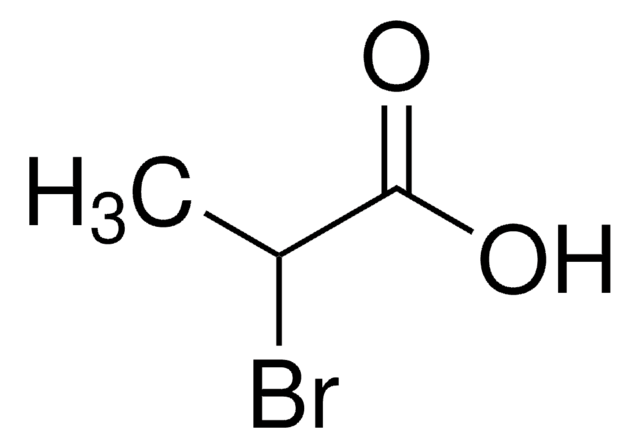
Linear Formula:
CH3CH(Cl)CO2H
CAS Number:
Molecular Weight:
108.52
Beilstein:
1720258
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
optical activity
[α]20/D +14°, neat
refractive index
n20/D 1.4345 (lit.)
bp
185-188 °C (lit.)
density
1.258 g/mL at 20 °C (lit.)
functional group
carboxylic acid
chloro
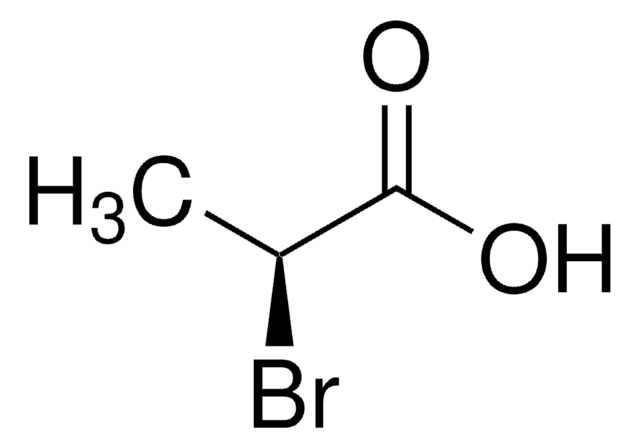
SMILES string
C[C@@H](Cl)C(O)=O
InChI
1S/C3H5ClO2/c1-2(4)3(5)6/h2H,1H3,(H,5,6)/t2-/m1/s1
InChI key
GAWAYYRQGQZKCR-UWTATZPHSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(R)-(+)-2-Chloropropionic acid can be used as a starting material in the synthesis of:
- Thiolactic acid, a key intermediate, which is used for the preparation of 2(R),5(S)-oxathiolanones by reacting with pivalaldehyde.
- (R)-2-chloropropionyl chloride, a key intermediate, which is utilized in the synthesis of (R)-4,5-dihydro-5-methylpyridazin-3(2H)-one derivative with pyrazolopyridine ring as a potential phosphodiesterase inhibitor.
- 2-Chloropropionamide derivatives as protein disulfide isomerase (PID) inhibitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cedric L Hugelshofer et al.
The Journal of organic chemistry, 84(21), 14069-14091 (2019-09-20)
We provide a full account of our synthetic studies targeting the hexacyclic calyciphylline B-type alkaloids, a subfamily of the Daphniphyllum natural products. Following an initial set of synthetic strategies focused on constructing the piperidine core of the calyciphylline B-type framework
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service