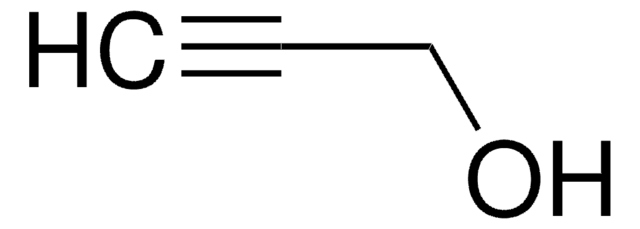302015
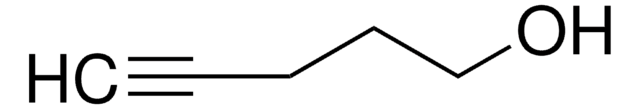
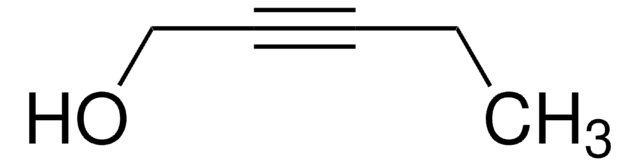
5-Hexyn-1-ol
96%
Synonym(s):
1-Hexyn-6-ol, 1-Hydroxy-5-hexyne, 5-Hexynol, 5-Hexynyl alcohol, 6-Hydroxy-1-hexyne
About This Item
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.450 (lit.)
bp
73-75 °C/15 mmHg (lit.)
density
0.89 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OCCCCC#C
InChI
1S/C6H10O/c1-2-3-4-5-6-7/h1,7H,3-6H2
InChI key
GOQJMMHTSOQIEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- cinnoline-fused cyclic enediyne
- llycopodium alkaloids, (+)-nankakurine A and (+)-nankakurine B
- 7-benzoyloxy-3-(2-nitrophenylseleno)-1,5-cyclodecadiyne
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Alkynes contain a highly versatile functional group that may be utilized for numerous reactions such as electrophilic additions of hydrogen, halogens, hydrogen halides, or water; metathesis; hydroboration; oxidative cleavage; C–C coupling; and cycloadditions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service