282766
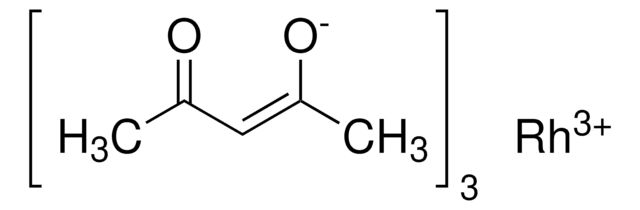
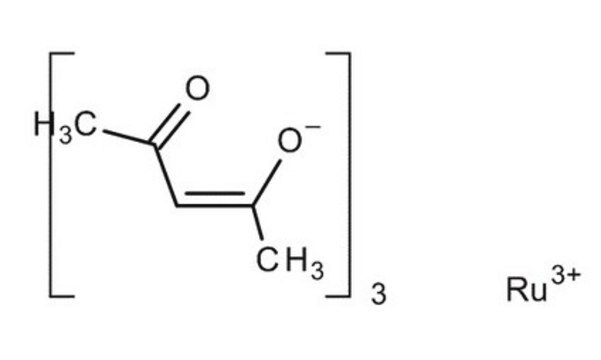
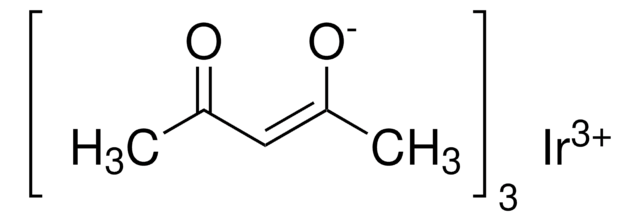
Ruthenium(III) acetylacetonate
97%
Synonym(s):
2,4-Pentanedione ruthenium(III) derivative, Ru(acac)3
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Ru(C5H7O2)3
CAS Number:
Molecular Weight:
398.39
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
mp
260 °C (dec.) (lit.)
SMILES string
CC(=O)\C=C(\C)O[Ru](O\C(C)=C/C(C)=O)O\C(C)=C/C(C)=O
InChI
1S/3C5H8O2.Ru/c3*1-4(6)3-5(2)7;/h3*3,6H,1-2H3;/q;;;+3/p-3/b3*4-3-;
InChI key
RTZYCRSRNSTRGC-LNTINUHCSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Ruthenium(III) acetylacetonate is a dark violet solid that exhibits high solubility in organic solvents. It exhibits fast kinetics for oxidation and reduction, facilitating efficient electrochemical reactions. It is widely used in the field of nanomaterial synthesis, solar cells, batteries, and supercapacitors.
Application
Ruthenium(III) acetylacetonate can be used:
- As an electrolyte in redox flow batteries. It helps to enhance the voltage efficiency of batteries.
- As a starting material to synthesize homogeneously dispersed Ru nanoparticles for super capacitor applications.
- As a precursor to synthesize ruthenium single atom multifunctional electrocatalyst that exhibits outstanding catalytic performance for zinc-air battery and overall water splitting reaction.
- To fabricate Ru2P anodic catalyst for polymer electrolyte fuel cells. It helps to improve hydrogen oxidation reaction performance.
- As a reliable and stable cathode interfacial layer to significantly improve solar cell efficiency and stability.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yuxiao Zhang et al.
Chemistry, an Asian journal, 14(2), 278-285 (2018-12-07)
Molybdenum disulfide (MoS2 ) has been regarded as a favorable photocatalytic co-catalyst and efficient hydrogen evolution reaction (HER) electrocatalyst alternative to expensive noble-metals catalysts, owing to earth-abundance, proper band gap, high surface area, and fast electron transfer ability. In order
Bih-Show Lou et al.
Scientific reports, 6, 19949-19949 (2016-01-29)
The synthesis of highly dispersed and stable ruthenium nanoparticles (RuNPs; ca. 2-3 nm) on porous activated carbons derived from Moringa Oleifera fruit shells (MOC) is reported and were exploited for supercapacitor applications. The Ru/MOC composites so fabricated using the biowaste carbon
Tuenissen, H.T. Elsevier, C.J.
Chemical Communications (Cambridge, England), 667-667 (1997)
Kaipeng Liu et al.
Nature communications, 11(1), 1263-1263 (2020-03-11)
Single-atom catalysts (SACs) have demonstrated superior catalytic performance in numerous heterogeneous reactions. However, producing thermally stable SACs, especially in a simple and scalable way, remains a formidable challenge. Here, we report the synthesis of Ru SACs from commercial RuO2 powders
Ming Zhao et al.
ACS nano, 13(6), 7241-7251 (2019-05-31)
Owing to their highly open structure and a large number of low-coordination sites on the surface, noble-metal nanoframes are intriguing for catalytic applications. Here, we demonstrate the rational synthesis of Ru cuboctahedral nanoframes with enhanced catalytic performance toward hydrazine decomposition.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 282766-1G | 4061826235782 |
| 282766-5G | 4061835508600 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service