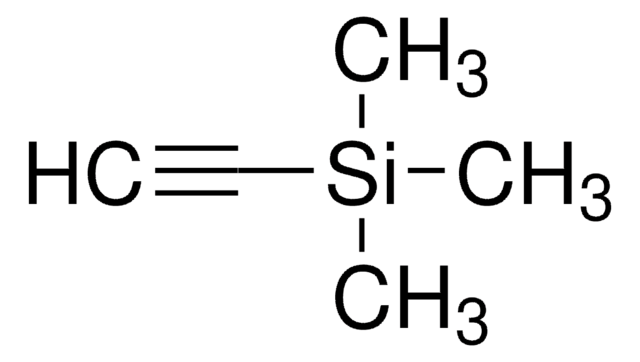244392
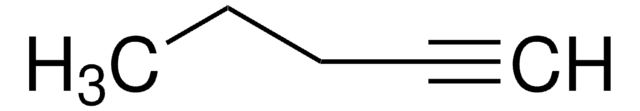
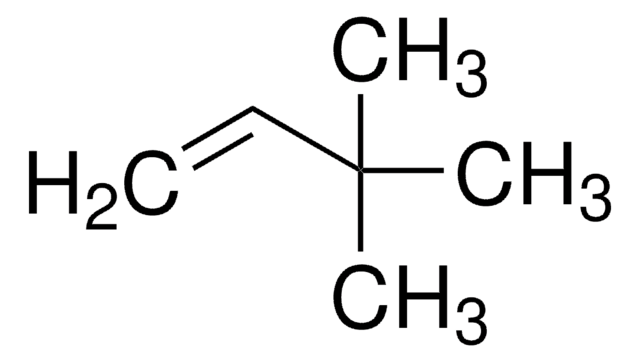
3,3-Dimethyl-1-butyne
98%
Synonym(s):
tert-Butylacetylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CC≡CH
CAS Number:
Molecular Weight:
82.14
Beilstein:
1697100
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
7.88 psi ( 20 °C)
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.374 (lit.)
bp
37-38 °C (lit.)
mp
−78 °C (lit.)
density
0.667 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)(C)C#C
InChI
1S/C6H10/c1-5-6(2,3)4/h1H,2-4H3
InChI key
PPWNCLVNXGCGAF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3,3-Dimethyl-1-butyne was used in the synthesis of erythro and threo isomers of B-(3,3-dimethyl-1,2-dideuterio-1-butyl)-9-BBN by hydroboration-deuteronolysis-hydroboration sequence.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
-21.1 °F
Flash Point(C)
-29.5 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karl Matos et al.
The Journal of organic chemistry, 63(3), 461-470 (2001-10-24)
Both erythro and threo isomers of B-(3,3-dimethyl-1,2-dideuterio-1-butyl)-9-BBN (6) were prepared from 3,3-dimethyl-1-butyne (4) through a hydroboration-deuteronolysis-hydroboration sequence employing first 9-BBN-H and then 9-BBN-D, or in reverse order, respectively. Employing the Whitesides protocol, the stereochemistry of B --> Pd alkyl group
E Fontana et al.
Current drug metabolism, 6(5), 413-454 (2005-10-27)
The inhibition of human cytochrome P450s (CYPs) is one of the most common mechanisms which can lead to drug-drug interactions. The inhibition of CYPs can be reversible (competitive or non-competitive) or irreversible. Irreversible inhibition usually derives from activation of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service