219576
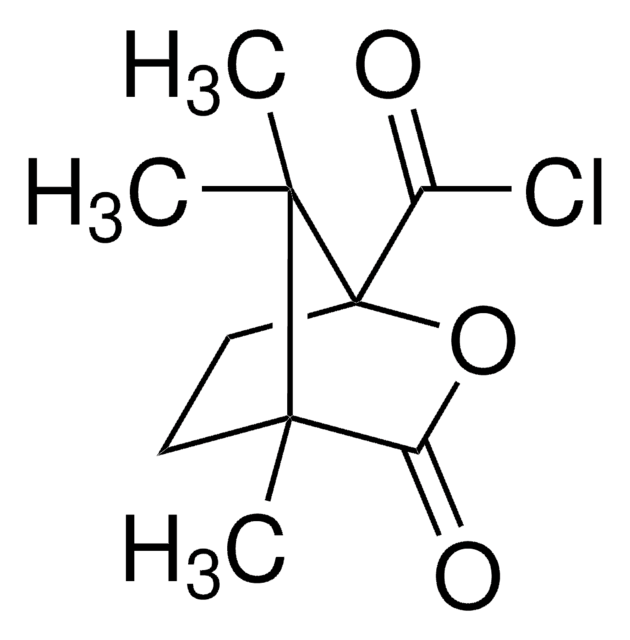
(1S)-(+)-10-Camphorsulfonyl chloride
97%
Synonym(s):
(+)-Camphor-10-sulfonyl chloride, (1S)-Camphor-10-sulfonic acid chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15ClO3S
CAS Number:
Molecular Weight:
250.74
Beilstein:
3205974
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
65-67 °C (lit.)
functional group
ketone
SMILES string
[H][C@@]12CC[C@@](CS(Cl)(=O)=O)(C(=O)C1)C2(C)C
InChI
1S/C10H15ClO3S/c1-9(2)7-3-4-10(9,8(12)5-7)6-15(11,13)14/h7H,3-6H2,1-2H3/t7-,10-/m1/s1
InChI key
BGABKEVTHIJBIW-GMSGAONNSA-N
Related Categories
Application
Chiral resolving agent. Used in the synthesis of nonpeptide oxytocin antagonists.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B E Evans et al.
Journal of medicinal chemistry, 35(21), 3919-3927 (1992-10-16)
The first nonpeptide antagonists of the neurohypophyseal hormone, oxytocin (OT) are described. Derivatives of the spiroindenepiperidine ring system, these compounds include L-366,509, an orally bioavailable OT antagonist with good in vivo duration. The potential use of these agents for treatment
Synthesis, 947-947 (1992)
T Jira et al.
Die Pharmazie, 48(11), 829-833 (1993-11-01)
Investigations on direct separation by RP-HPLC of selected enantiomeric beta-adrenergic active agents are described. R- and S-1-phenylethylisocyanate (PEIC) as well as (1S)-(+)-campher-10-sulfonylchloride (CSC) are used for the derivatization of the compounds. Correlations between chromatographic data and various influences (pH, temperature
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 219576-100G | 4061838776037 |
| 219576-25G | 4061838776044 |
| 219576-5G | 4061833037867 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service