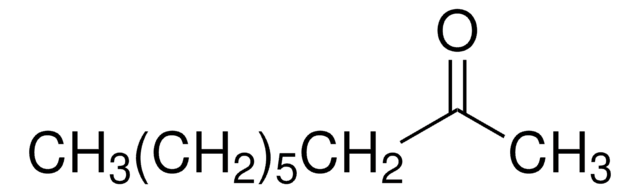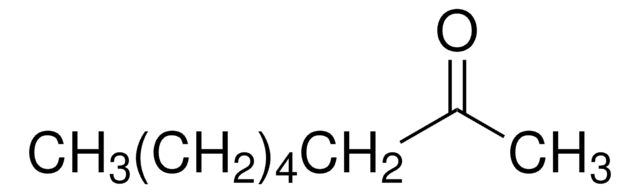136913
3-Octanone
≥98%
Synonym(s):
Ethyl amyl ketone, Ethyl pentyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4COC2H5
CAS Number:
Molecular Weight:
128.21
Beilstein:
1700021
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.415 (lit.)
bp
167-168 °C (lit.)
density
0.822 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CCCCCC(=O)CC
InChI
1S/C8H16O/c1-3-5-6-7-8(9)4-2/h3-7H2,1-2H3
InChI key
RHLVCLIPMVJYKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Octanone is the common metabolite produced by some fungal species.
Application
3-Octanone was used to study the effect TiO2 photocatalyst on the rate and the ratio of products generated in photocatalytic oxidation of 3-octanone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
123.8 °F - closed cup
Flash Point(C)
51 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Effects of water, salt water, and silicone overcoating of the TiO2 photocatalyst on the rates and products of photocatalytic oxidation of liquid 3-octanol and 3-octanone.
Sunada F and Heller A.
Environmental Science & Technology, 32(2), 282-286 (1998)
A L Sunesson et al.
The Annals of occupational hygiene, 40(4), 397-410 (1996-08-01)
Two fungal species commonly found in indoor environments, Penicillium commune and Paecilomyces variotü, were cultivated on pine wood and on a combination of gypsum board and mineral wool. Air from the cultures was adsorbed on Tenax TA and analysed using
D Tuma et al.
International journal of food microbiology, 8(2), 103-119 (1989-05-01)
Western hard red spring wheat, stored at 20 and 25% moisture contents for 10 months during 1985-86, was monitored for biotic and abiotic variables in 10 unheated bins in Winnipeg, Manitoba. The major odor volatiles identified were 3-methyl-1-butanol, 3-octanone and
Martin J Schnermann et al.
Proceedings of the National Academy of Sciences of the United States of America, 107(14), 6158-6163 (2010-03-25)
Golgi-modifying properties of the spongian diterpene macfarlandin E (MacE) and a synthetic analog, t-Bu-MacE, containing its 2,7-dioxabicyclo[3.2.1]octan-3-one moiety are reported. Natural product screening efforts identified MacE as inducing a novel morphological change in Golgi structure defined by ribbon fragmentation with
Marek Nemcovic et al.
FEMS microbiology letters, 284(2), 231-236 (2008-05-31)
Light and starvation are two principal environmental stimuli inducing conidiation in the soil micromycete Trichoderma spp. We observed that volatiles produced by conidiating colonies of Trichoderma spp. elicited conidiation in colonies that had not been induced previously by exposure to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service