129631
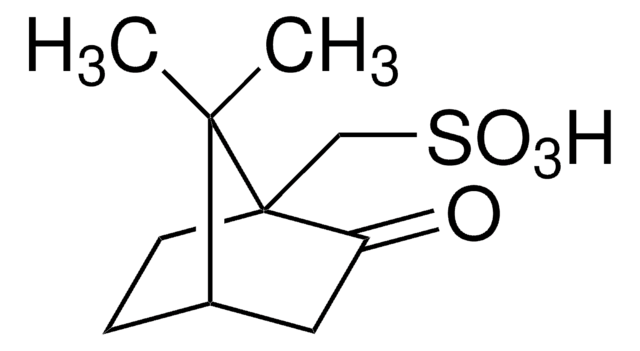
2-Mesitylenesulfonic acid dihydrate
97%
Synonym(s):
2,4,6-Trimethylbenzenesulfonic acid dihydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3C6H2SO3H · 2H2O
CAS Number:
Molecular Weight:
236.29
Beilstein:
1961501
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
74-78 °C (lit.)
functional group
sulfonic acid
storage temp.
2-8°C
SMILES string
[H]O[H].[H]O[H].Cc1cc(C)c(c(C)c1)S(O)(=O)=O
InChI
1S/C9H12O3S.2H2O/c1-6-4-7(2)9(8(3)5-6)13(10,11)12;;/h4-5H,1-3H3,(H,10,11,12);2*1H2
InChI key
LIKWMDGAWXCECN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Mesitylenesulfonic acid dehydrate was used in the preparation of tert-Butyl (2S,4S)-N,N-di-tert-butoxycarbonyl-5-fluoroleucine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jean-Damien Charrier et al.
Organic & biomolecular chemistry, 2(4), 474-482 (2004-02-11)
Syntheses of (2S,4S)- and (2S,4R)-5-fluoroleucine, and, and of (2S,4S)-[5,5-(2)H(2)]-5-fluoroleucine, have been completed. The methodology allows these compounds to be prepared in sufficient quantities for incorporation by solid-state protein synthesis into strategic sites in proteins for folding studies. X-ray structures of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service