101273
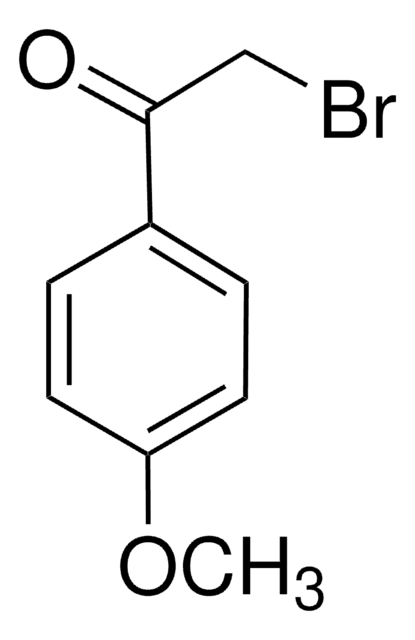
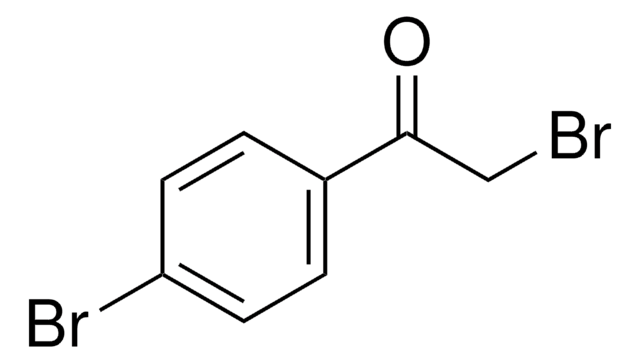
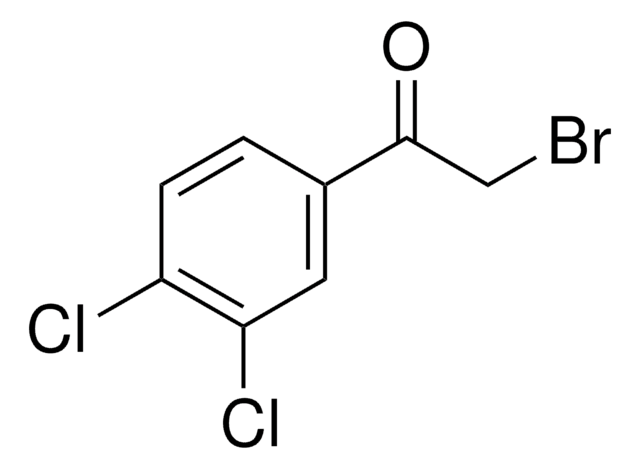
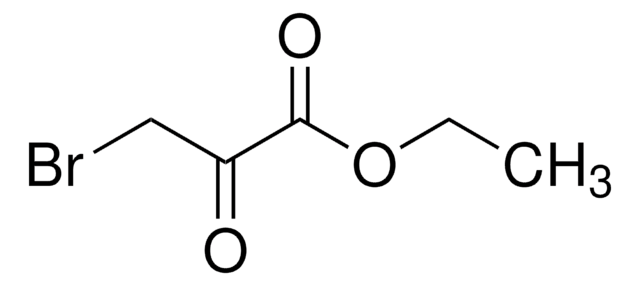
2-Bromo-4′-chloroacetophenone
98%
Synonym(s):
ω-Bromo-4-chloroacetophenone, 4-Chlorophenacyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
ClC6H4COCH2Br
CAS Number:
Molecular Weight:
233.49
Beilstein:
607603
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
93-96 °C (lit.)
functional group
bromo
SMILES string
Clc1ccc(cc1)C(=O)CBr
InChI
1S/C8H6BrClO/c9-5-8(11)6-1-3-7(10)4-2-6/h1-4H,5H2
InChI key
FLAYZKKEOIAALB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniel I Perez et al.
Journal of medicinal chemistry, 54(12), 4042-4056 (2011-04-20)
Development of kinase-targeted therapies for central nervous system (CNS) diseases is a great challenge. Glycogen synthase kinase 3 (GSK-3) offers a great potential for severe CNS unmet diseases, being one of the inhibitors on clinical trials for different tauopathies. Following
Shigeo Hayashi et al.
Journal of enzyme inhibition and medicinal chemistry, 29(6), 846-867 (2014-02-13)
Because of the pivotal role of cyclooxygenase (COX) in the inflammatory processes, non-steroidal anti-inflammatory drugs (NSAIDs) that suppress COX activities have been used clinically for the treatment of inflammatory diseases/syndromes; however, traditional NSAIDs exhibit serious side-effects such as gastrointestinal damage
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service