Fullerenes for Bioscience & Photovoltaic Applications
Michael D. Diener
TDA Research
Condensed Fullerene
Fullerenes in Photovoltaics
Fullerenes in Biosciences
Small-Cap Fullerenes
Introduction
Twenty-five years ago, it was discovered that carbon vapor preferentially condenses into an exceedingly stable cluster with 60 carbon atoms (C60), and it was hypothesized that the 60 atoms were arranged as the vertices of a truncated icosahedron, aka a soccer ball.1 The carbon cluster was named Buckminsterfullerene, after the architect of building structures based on geodesic domes. Five years later, using methods for the synthesis of Buckminsterfullerene in milligram quantities it was revealed that not only are the C60 clusters stable in air, but also soluble in aromatic solvents.2 Now rightfully thought of as molecules instead of meta-stable clusters, it became clear that C60 was not the only “fullerene,” but there exist a whole set of stable, soluble, all-carbon molecules with C70 (Product No. 482994), C76 (Product No. 482951), C78 (Product No. 482978), C84 (Product No. 482986) and even over 100 atoms per molecule (and no hydrogen atoms). In nature, fullerenes have been detected in interstellar dusts and in the ground near meteorite impacts. Over time, their synthesis from carbon vapors progressed from grams to kilograms, and eventually a technique for their synthesis in sooting flames lead to their production in tons.3 Thus, what Prof. Eiji Osawa once described as a chicken and egg problem – there are no commercial applications for fullerenes because there is no large-scale production, and there is no large-scale production because there are no commercial applications – has been solved.
The development of fullerenes for various commercial applications is currently at the tipping point. Sporting goods, such as badminton racquets with fullerenes in the polymer matrix composite, and cosmetics, such as the Vitamin C60 skin creams, are available to consumers in select markets. Fullerenic materials are already being investigated for a wide variety of purposes currently filled by other forms of carbon, including catalyst supports, battery anodes, and proton transport membranes. However, by far the most publicized and most promising developments in fullerene research are in the fields of organic electronics and bioscience.
Fullerenes in Photovoltaics
Fullerenes have an extremely low reorganization energy following electron transfer, a property that makes them one of the most useful electron acceptor molecules in organic electronics.4 They are also n-type semiconductors (with band gap = 2.3eV), making them a good counterpart to the numerous good p-type organic semiconductors. Their (nearly) spherical structure allows electron transfer to occur in any spatial direction without preference, and their electron mobility (µe= 0.1cm2/Vs)is excellent for an organic material. C60 or C70 can be used to make transistors with excellent performance in inert environments. Also, many C60 anion (“fulleride”) salts are superconductors with respectable transition temperatures (~30 K).
Fullerenes have also found application in the vibrant research field of organic photovoltaics (OPVs). Significant progress has been made in the power conversion efficiency of the devices, as well as the development of more stable plastic solar cell architectures.5 Fullerenes serve as the electron acceptor in almost all reported high-performance devices (Figure 1). C60 is the standard for vacuum-deposited OPVs, while the more soluble PCBM (Product No. 684449) is the standard for solution-deposited OPVs. Further performance enhancements are usually possible with PC70BM (Product No. 684465). The band energies of fullerenes line up moderately well with the bands of the photon-absorbing/electron-donating molecules, leading to reasonably efficient exciton dissociation and charge transfer. Substantial growth in this rapidly advancing technology is forecast for the near future.

Fullerenes in Bioscience
The ability to add an electron and then remove it with almost no energy cost, also lies at the heart of primary biological application of the fullerenes: as antioxidants. Certain water-soluble fullerenes catalytically remove potentially harmful oxidant species, acting as a synthetic analog to naturally-occurring superoxide dismutase. Their antioxidant behavior, or perhaps the antiproliferative result of removing free radicals involved in the cell cycle, has extended in the lifespan of geriatric mice, and has also improved their advanced age cognition.6 As a result, water-soluble fullerenes, such as C60 Pyrrolidine tris-acid (Product No. 709085), are explored for applications in therapies against a variety of degenerative conditions involving an excess of reactive oxygen species.
Fullerenes are also being developed for drug delivery applications. They have a rich and well-documented organic chemistry, and researchers have developed many versatile routes for the formation of covalent bonds to fullerenes. Various therapeutic agents have been covalently attached to fullerenes using these methods, allowing for the tailoring of their pharmacokinetic properties. 7 While many nanomaterials are being developed for the same purpose, the compact size of fullerenes (~1 nm diameter, illustrated in Figure 2) compares favorably to nanoparticles (10 – 100 nm diameter) in terms of enhancing solubility and avoiding aggregation during delivery. Moreover, C60 is a specific, isomerically pure molecule, and covalently-bonded derivatives can be readily characterized by standard analytical chemistry techniques.
Figure 2.Hypothetical fullerene antibody conjugate constructed from a C82 carboxymetallofullerene and a chimeric B72.3 Fab’ antibody fragment. The B72.3 x-ray structure is from the national protein databank entry 1BBJ.1
The cavity in the middle of the carbon cage is another inherent and unique property of fullerenes. This space can accommodate atoms, or clusters of a few atoms at most. Once the atoms are inside the fullerene, only extremely energetic events, such as nuclear recoil following alpha decay, will allow them to escape. As a result, these “endohedral fullerenes”, fullerenes with one or more atoms inside, are being developed as imaging agents. The imaging is still enabled by the encapsulated atom, but the biodistribution is now dictated by the fullerene and the covalently bonded groups attached to its exterior. Magnetic resonance imaging (MRI) contrast agents are the leading example of fullerene imaging agent development , but fullerenes transporting radioactive isotopes are also being explored. A number of fullerene derivatives have also been investigated as sensitizers for photodynamic therapy, and aggregates for DNA transfection.
Although preliminary reports of significant toxicity from aqueous suspensions of C60 aggregates (“nano-C60”) garnered wide attention in the secondary press, subsequent investigations conclusively demonstrated that the toxicity was due to a by-product of the method of forming the aqueous suspension, not the fullerenes themselves.9 Nevertheless, fullerene derivatives bearing specific chemical groups can disrupt cell walls and be used as antibacterial and antiviral agents.
Small-Gap Fullerenes
In partnership with TDA Research, we are pleased to introduce purified Small-Gap Fullerenes (Product No. 707503).11 Until now, all commercially available fullerenes were recovered from the non-fullerenic carbon matrix by solvent extraction. However, the solvent extraction process is relatively inefficient, as many fullerenes polymerize into an insoluble state. The polymerization can be mediated by oxygen, but can also occur spontaneously. Fullerenes with small energy differences between filled and unfilled electronic states of the individual fullerene molecules (“small gap fullerenes”) are especially susceptible to such spontaneous polymerization,12 and are therefore not recovered by solvent extraction. The percentage of isomers of a certain cage size which are “small gap” increase dramatically as the cage size increases; thus, solvent-extracted fullerenes have few giant fullerenes, while small gap fullerenes (SGFs) are rich in giant fullerenes larger than C84.11 Despite their relative abundance, SGFs are usually therefore discarded along with the non-fullerenic carbon matrix.
Figure 3 compares the fullerene distribution of the raw combustion soot with that of the SGF fullerenes as well as that of conventional solvent-extracted fullerenes. The mass spectra were generated by time-of-flight mass spectrometry (TOF-MS) using a 355 nm laser pulse to desorb the fullerenes and a 118 nm laser pulse to ionize the fullerenes. Since all fullerenes are ionized with a single 118 nm photon, the distribution depicted is accurate for the fullerenes in the gas phase, although not all fullerenes may be desorbed with equal efficiency. Analysis of the peak heights shows that 60% of the fullerenes present are larger than C70, and 50% of the fullerenes present are larger than C84. The C60 and C70 remaining appear to have been bound up in the polymers and therefore not extracted by solvents. SGFs are extremely rich in fullerene cage sizes and isomers which are not present in conventional solvent-extracted fullerenes, including, for example, C74.
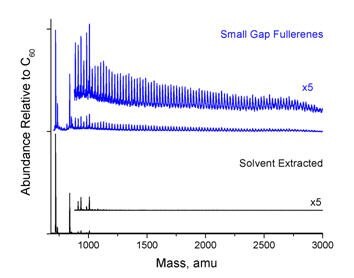
Figure 3.Top: Mass distribution of the small gap fullerene product. Bottom: distribution of fullerenes recovered by conventional solvent-extraction.
SGFs may find application both as fullerenic materials and, following covalent chemical modification, as fullerene derivatives. As received, or following thermal treatment to covert residual hydrocarbon impurities to carbon, SGFs form a novel nanostructured carbon material, which may be useful as a sorbent, catalyst support, or additive to polymeric matrices. Following reduction into a suitable solvent, where the fullerene anions are discreet soluble molecules, SGF films can be electroplated onto a number of substrates to give novel carbon coatings (Figure 4). For example, pressed pellets of SGFs have a bulk conductivity of ~10-4 S/cm.

Figure 4.Electroplated SGF film on Ti foil.
While the chemistry of SGFs remains in its infancy, certain reactions known for C60 also derivatize SGFs, leading to soluble products.13 For example, water-soluble polyhydroxylated SGFs (Product No. 707481) can be produced, which may be useful as water-soluble antioxidants/antiproliferatives. Alternately, organic-soluble SGF esters (Product No. 707473) as shown in Figure 5 can be produced, which may find applications in solution-processable organic electronics as n-type materials.

Figure 5.Left: Water-soluble polyhydroxylfullerene SGF example. Middle: Organic Soluble SGF ester. Right: C74, an example of a fullerene present within the un-functionalized SGF.
The SGFs offered here represent the third carbon material, after graphite and diamond, and, considering the technological importance of the first two, a very bright future is forecast for the SGFs as well.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?