Impact of Mobile Phase Additives on LC-MS Sensitivity, Demonstrated using Spice Cannabinoids
Xiaoning Lu, Craig Aurand, David, S Bell
Reporter US, Volume 30.3
The article stresses the importance of using the highest quality solvents and reagents, along with high efficiency HPLC columns and effective sample prep methods, to provide the best MS data.
Introduction
Part 1 of our Topics in LC-MS, published in Reporter 30.2, looked at leveraging column selectivity in developing robust LC-MS methods1. Part 2 examines the important role of mobile phase components, specifically the ionic modifiers. In this article, the LC-MS/ MS optimization for analysis of spice cannabinoids is demonstrated using a range of mobile phase additives for increased analyte response and selectivity. A four-pronged approach leveraging HPLC column selectivity, solvent purity, effective sample prep, and reference standards, was used to develop a method to rapidly isolate and identify spice cannabinoids from plasma.
Background
Optimized mobile phases for LC-UV methods are not necessarily transferable to methods employing MS detection. Usually when developing LC-UV methods the main consideration is achieving adequate retention and resolution of the analytes while maintaining low UV background. However, for LC methods using electrospray ionization (ESI) detection, one not only has to consider retention and resolution, but also how the mobile phase components impact ionization. LC-ESI mobile phase components also must be volatile, so they are efficiently vaporized in the ESI source of the MS inlet, and free of impurities that can contribute to the background, reduce sensitivity by forming adducts, or otherwise impact the sensitivity and overall quality of the MS experiment. Typical mobile phase modifiers used in LC-MS include ammonium formate and ammonium acetate buffers and formic, acetic, and trifluoroacetic acids.
Spice Cannabinoids: Current Analytical Interest
Synthetic cannabinoids (Spice, Figure 1) are a relatively new type of designer drug used as a pseudo-legal means to get a cannabistype high2. In early 2011, the US Drug Enforcement Agency (DEA) placed several of the most popular synthetic cannabinoids such as JWH-018 and JWH-073 on their Schedule 1 list, making the possession or consumption of these compounds illegal. However, new synthetic cannabinoids are continually being introduced as suppliers tweak the molecular structures. The ability to rapidly and reliably identify the continually changing population of these compounds in the blood or urine of suspected users is a significant analytical challenge facing forensic chemists.
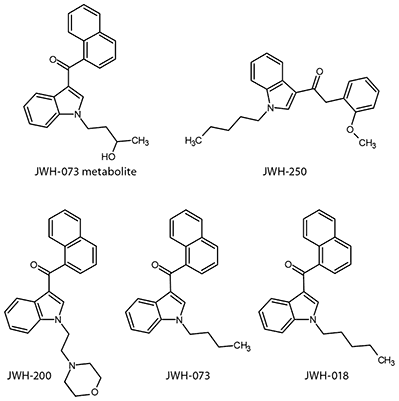
Figure 1. Representative Spice Compounds
Study of the Mobile Phase Ionic Modifier on MS Signal
Method development employed screening various Ascentis® Express phases. The Ascentis Express F5 gave the best resolution of the five spice cannabinoids under isocratic conditions. Therefore, it was used for the experiments to determine which mobile phase modifiers enabled the highest ESI-MS response for the five spice cannabinoids. Mobile phases comprising 5 mM ammonium acetate, 5 mM ammonium formate, 0.05% acetic acid, or 0.05% formic acid in 50:50 water:acetonitrile were evaluated for chromatographic resolution along with ESI-MS response.
Impact of Solvents and Additives on Resolution and Relative Response
Figure 2 shows the resulting chromatograms of the additive study. Resolution of the five compounds was obtained under each of the four conditions tested. Elution orders were comparable except for one compound, JWH-200, which had longer retention under the acidic conditions. (This is possibly due to the known ion exchange character of the F5 and the basic nature of the morpholine functional group of JWH-200. Further experiments are required to fully elucidate the retention mechanism.)
Conditions
| column: | Ascentis Express F5, 5 cm x 2.1 mm, 2.7 µm (Product No. 53567-U) |
| mobile phase: | (50:50) acetonitrile:water containing various ionic additives as shown in figure |
| flow rate: | 0.3 mL/min |
| temp.: | 35 °C |
| pressure (column): | 1296 psi (89.4 bar) |
| detector: | MS, ESI(+), MRM, m/z 344/155 (JWH-073 metabolite), 385/155 (JWH-200), 336/121 (JWH-250), 328/155 (JWH-073), and 342/155 (JWH-018) |
| injection: | 2 µL |
| sample: | 5 ng/mL each compound in 50/50 water:methanol |
| system: | Agilent 1100 HPLC, 3200 QTRAP (AB/Sciex) |
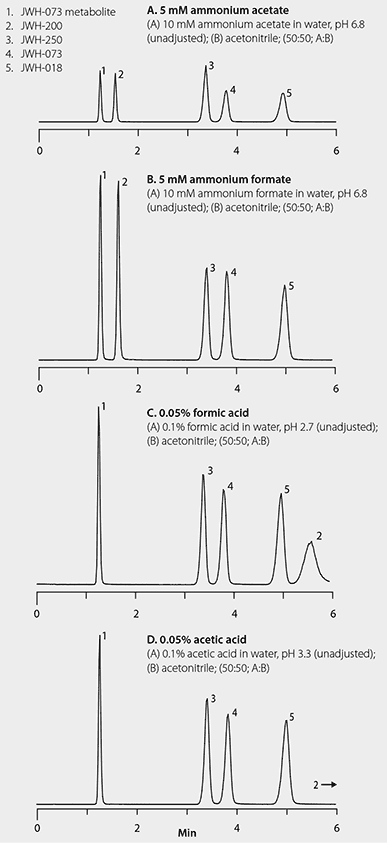
Figure 2.LC-MS/MS Resolution and Response of Spice Cannabinoids with Various Ionic Mobile Phase Additives
Regarding analyte response, the formate systems gave higher analyte response than the acetate systems, with the ammonium formate conditions giving the overall best results when retention of JWH-200 is taken into account (Table 1). Although coelution was not a problem in this example; for LC-MS separations, it is generally advisable to select a method based on analyte response rather than chromatographic resolution when analytes are readily discriminated by specific MS/MS transitions, as is the case with these spice compounds.
Relative Response (Peak Height) in ESI(+) Mode of the Spice Compounds under Different Mobile Phase Conditions |
|---|
Adduct Formation and Solvent Purity
Adduct formation is also an important consideration when optimizing LC-MS methods for sensitivity. Adduct formation of the target analyte, primarily with sodium and potassium in ESI(+), can sacrifice the formation of target protonated analyte ions, thus decreasing the overall sensitivity of the method and complicating the spectrum. Previous studies have discussed the adduct formation phenomenon in detail, stressing the importance of using solvents and eluent additives that are free of unwanted ions3.
Analysis of Spice Compounds in Plasma Samples
The optimized MS method on standards was then used on a spiked plasma sample. Endogenous proteins and phospholipids were first removed using HybridSPE®- Phospholipid. Details of the sample preparation method and mechanism of the HybridSPE-Phospholipid can be found in reference 4. Figure 3 shows the resulting LC-MS/ MS chromatogram of the five spice compounds extracted from plasma and resolved on the Ascentis Express F5 column.

Figure 3. LC -MS/MS Analysis of Spice Compounds from Plasma on Ascentis Express F5 after SPE using HybridSPE- Phospholipid
Summary
Special consideration of mobile phase components must be made when optimizing methods for LC-MS applications. Using the socially problematic spice cannabinoids as the test case, the study reported here demonstrates the impact of various mobile phase modifiers on the separation, with the formate modifiers outperforming acetate in terms of MS signals (or sensitivity) and chromatographic resolution. This study is prime example of the benefit of using high-purity solvents with the HybridSPE-Phospholipid 96-well plates for effective sample cleanup, Ascentis Express UHPLC columns for rapid, efficient separations, and Cerilliant standards for reliable characterization and quantification of analytes in biological matrices.
Conclusion
From these results, it can be concluded that this SPE protocol using HybridSPE-Phospholipid 96-well plates followed by LC-MS/MS is useful for global metabolome analysis by fractionation, here into non-PL and PL metabolites. Therefore, the multipurpose use of HybridSPE-Phospholipid products commonly used for interference removal has been demonstrated.
Complete details on this research and the PLs detected and confirmed by MS/MS, can be found in the reference.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?