Enzymatic Assay of Glucose-6-Phosphate Dehydrogenase (EC 1.1.1.49)
1. Objective
To standardize a procedure for the enzymatic determination of glucose-6-phosphate dehydrogenase.
2. Scope
This procedure applies to most products that have a specification for glucose-6-phosphate dehydrogenase by enzymatic determination. This assay is not for glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides.
3. Definitions
3.1. Purified Water - water from a deionizing system, resistivity > or = 18MΩ•cm @ 25 ºC
3.2. Unit Definition - One unit will oxidize 1.0 μmol of D-glucose-6-phosphate to 6-phospho-D-gluconate per minute in the presence of β-NADP at pH 7.4 at 25 °C.
3.3. G-6-PDH - Glucose-6-Phosphate Dehydrogenase
3.4. β-NADP - β-Nicotinamide Adenine Dinucleotide Phosphate, Oxidized Form
3.5. β-NADPH - β-Nicotinamide Adenine Dinucleotide Phosphate, Reduced Form
4. Discussion

5. Responsibilities
Analytical services personnel should follow this protocol as written.
6. Safety
Refer to the Safety Data Sheet (SDS) for hazards and appropriate handling precautions.
7. Procedure
7.1. CONDITIONS
T = 25 °C, pH = 7.4, absorbance at 340 nm, Light path = 1 cm
7.2. METHOD
Spectrophotometric Rate Determination
7.3. REAGENTS
7.3.1. 250 mM Glycylglycine (Buffer)
Prepare a 33 mg/mL solution in purified water using Glycylglycine, Free Base, Product No. G1002. Adjust to pH 7.4 at 25 °C with 1 M NaOH or 1 M HCl.
7.3.2. 60 mM D-Glucose 6-Phosphate Solution (G-6-P)
Prepare 17 mg/mL in purified water using D-Glucose-6 Phosphate, Monosodium Salt, Product No. G7879.
7.3.3. 20 mM β-Nicotinamide Adenine Dinucleotide Phosphate Solution (β-NADP)
Prepare 15.4 mg/mL in purified water using β-Nicotinamide Adenine Dinucleotide Phosphate, Sodium Salt, Product No. N0505.
7.3.4. 300 mM Magnesium Chloride Solution (MgCl2)
Prepare 0.3 mL/mL in purified water using 1.0 M Magnesium Chloride Solution, Product No. M1028.
7.3.5. Glucose-6-Phosphate Dehydrogenase Enzyme Solution (Enzyme)
Immediately before use, prepare 0.3-0.6 units/mL in cold buffer (Reagent 7.3.1).
7.4. PROCEDURE
7.4.1. Prepare a reaction cocktail by pipetting (in milliliters) the following reagents into a suitable container:
7.4.2. Mix and equilibrate to 25 °C. Adjust the pH of the reaction cocktail to 7.4 with 1 M NaOH or 1 M HCl.
7.4.3. Pipette (in milliliters) the following reagents into suitable cuvettes:
7.4.4. Equilibrate to 25 °C. Monitor the A340nm until constant, using a suitably thermostatted spectrophotometer. Then add:
7.4.5. Immediately mix by inversion and record the increase in A340nm for approximately 10 minutes. Obtain the ΔA340nm/minute using the maximum linear rate for both the Test and Blank using a minimum of 4 data points over a one minute time interval.
7.5. CALCULATIONS
7.5.1

Where:
3 = Total volume (in milliliters) of assay
df = Dilution factor
6.22 = Millimolar extinction coefficient of β-NADPH at 340 nm
0.1 = Volume (in milliliters) of enzyme used
7.5.2
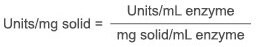
7.5.3

7.6. FINAL ASSAY CONCENTRATION
In a 3.00 mL reaction mix, the final concentrations are 50 mM glycylglycine, 2 mM D-glucose-6-phosphate, 0.67 mM β-nicotinamide adenine dinucleotide phosphate,10 mM magnesium chloride, and 0.03 - 0.06 units glucose-6-phosphate dehydrogenase.
8. Reference
Noltmann, E.A., Gubler, C.J., and Kuby, S.A. (1961) Journal of Biological Chemistry 236, 1225- 1230.
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?