442232-U
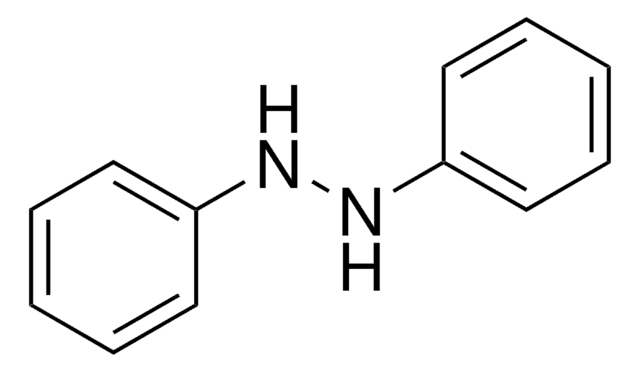
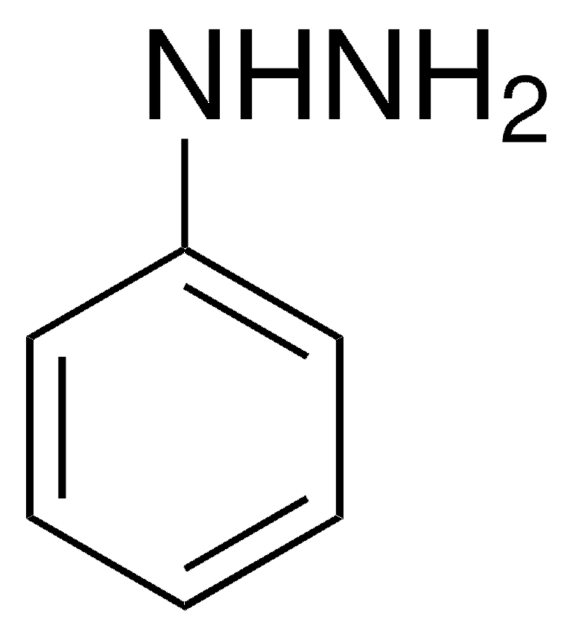
1,2-Diphenylhydrazine
analytical standard, ampule of 100 mg
Sinonimo/i:
Hydrazobenzene, N,N′-Diphenylhydrazine, N,N′-Bianiline, NSC 3510
About This Item
Prodotti consigliati
Grado
analytical standard
CdA
current certificate can be downloaded
Confezionamento
ampule of 100 mg
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Punto di fusione
123-126 °C (lit.)
applicazioni
cleaning products
cosmetics
environmental
food and beverages
personal care
Formato
neat
Temperatura di conservazione
2-30°C
Stringa SMILE
N(Nc1ccccc1)c2ccccc2
InChI
1S/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
YBQZXXMEJHZYMB-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Insertion reactions with organometallic tantalum complexes
- Reduction reactions catalyzed by titanium(III) trichloride yielding amines
- Studying the mechanism of hydrazobenzene rearrangement
- Reaction with N-heterocyclic stable silylene
- Synthesis of dimanganese amide hydrazide cluster complexes
- Iron-mediated hydrazine reductions yielding iron arylimide cubanes
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Codice della classe di stoccaggio
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.