1086222
USP
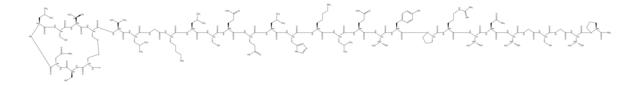
Calcitonin Salmon Related Compound B
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
Calcitonin salmon-glycine
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C147H242N44O50S2
Numéro CAS:
Poids moléculaire :
3489.89
Code UNSPSC :
41116107
Produits recommandés
Qualité
pharmaceutical primary standard
Agence
USP
Fabricant/nom de marque
USP
Application(s)
pharmaceutical
Format
neat
Température de stockage
2-8°C
Description générale
Calcitonin Salmon is a polypeptide that has the same sequence as that of the hormone that regulates calcium metabolism and is secreted by the ultimobranchial gland of salmon. It is produced from either synthetic processes or microbial processes using recombinant DNA (rDNA) technology. The host cell-derived protein content and the host cell- or vector-derived DNA content of Calcitonin Salmon produced from an rDNA process are determined by validated methods. This product contains NLT 90.0% and NMT 105.0% of calcitonin salmon, calculated on an acetic acid-free and anhydrous basis.
Calcitonin Salmon USP reference standard is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Calcitonin Salmon USP reference standard is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Calcitonin Salmon Related Compound B USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also used to prepare system suitability solutions according to the USP monographs. Further information is available in monograph, Calcitonin Salmon, USP43-NF38 – 670 of United States Pharmacopeia (USP).
Also used to prepare system suitability solutions according to the USP monographs. Further information is available in monograph, Calcitonin Salmon, USP43-NF38 – 670 of United States Pharmacopeia (USP).
This product is intended for use as a reference standard in pharmaceutical research and development. This product is for test and assay use only. It is not tested for animal or human consumption, clinical testing, or therapeutic use.
Remarque sur l'analyse
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Autres remarques
Sales restrictions may apply.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique