T1952
Trichostatin A, Ready Made Solution
5 mM in DMSO, from Streptomyces sp.
Synonyme(s) :
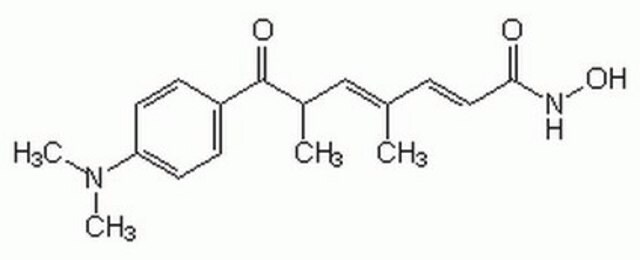
TSA, Trichostatin A, [R-(E,E)]-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide
About This Item
Produits recommandés
Source biologique
Streptomyces sp.
Niveau de qualité
Pureté
≥98% (HPLC)
Forme
DMSO solution
Concentration
5 mM in DMSO
Technique(s)
cell culture | mammalian: suitable
Spectre d'activité de l'antibiotique
fungi
neoplastics
Mode d’action
enzyme | inhibits
Conditions d'expédition
dry ice
Température de stockage
−20°C
InChI
1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+
Clé InChI
RTKIYFITIVXBLE-WKWSCTOISA-N
Description générale
Application
- as a histone deacetylase inhibitor to study its effect on transcriptome changes by stem cell testing
- to inhibit histone deacetylase (HDAC) class I, II, or III in primary pituitary cell cultures and to investigate insulin control of endogenous human growth hormone gene (hGH)
- to treat cells for the HDAC inhibition experiments
Actions biochimiques/physiologiques
Produit(s) apparenté(s)
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique