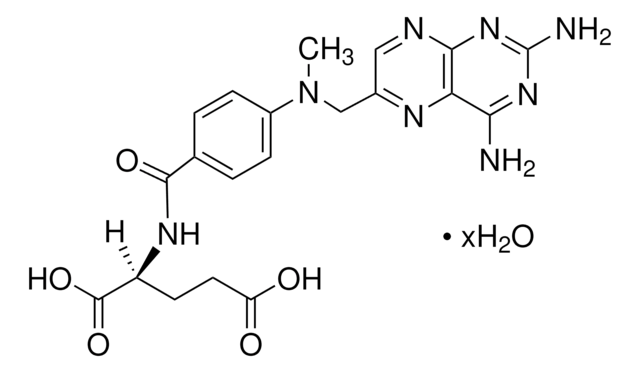
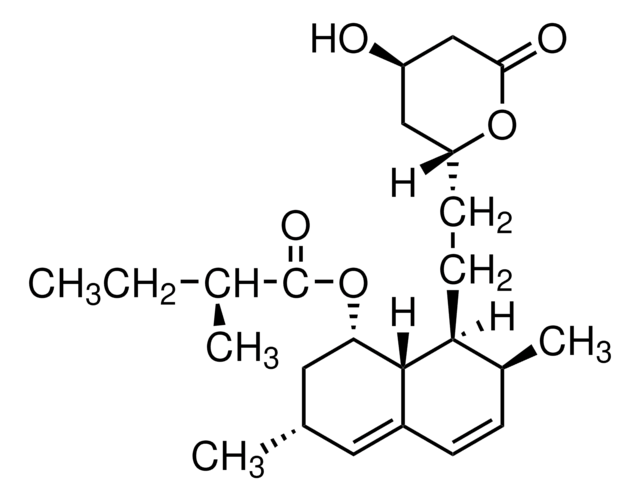
S4194
SR 12813
≥98%, solid
Synonyme(s) :
Tetraethyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethenyl-1,1-bisphosphonate
About This Item
Produits recommandés
Source biologique
synthetic (organic)
Niveau de qualité
Essai
≥98%
Forme
solid
Solubilité
DMSO: ≥10 mg/mL
H2O: insoluble
Auteur
GlaxoSmithKline
Chaîne SMILES
CCOP(=O)(OCC)C(=C/c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)\P(=O)(OCC)OCC
InChI
1S/C24H42O7P2/c1-11-28-32(26,29-12-2)21(33(27,30-13-3)31-14-4)17-18-15-19(23(5,6)7)22(25)20(16-18)24(8,9)10/h15-17,25H,11-14H2,1-10H3
Clé InChI
YQLJDECYQDRSBI-UHFFFAOYSA-N
Application
Actions biochimiques/physiologiques
Caractéristiques et avantages
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique