S2076
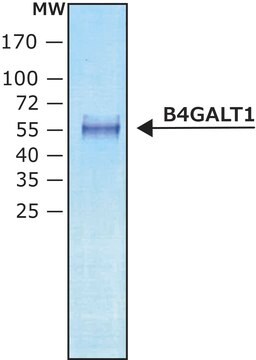
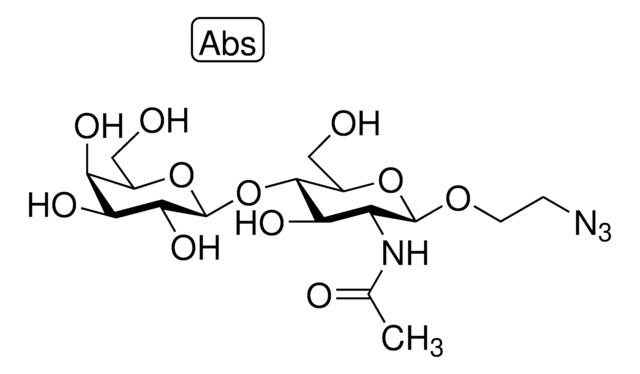
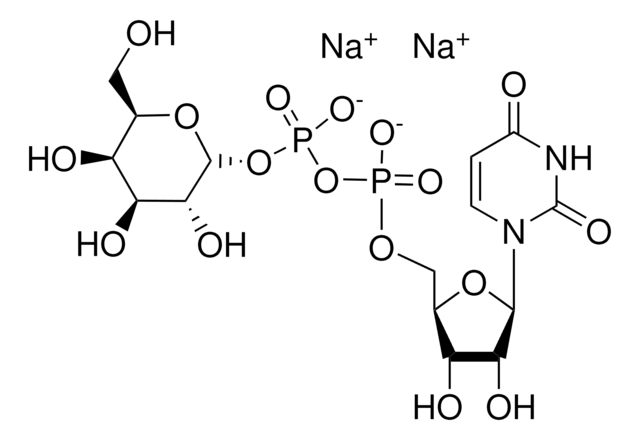
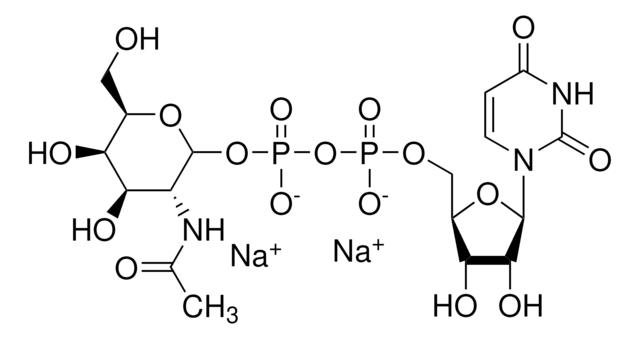
α-2,6-Sialyltransferase from Photobacterium damsela
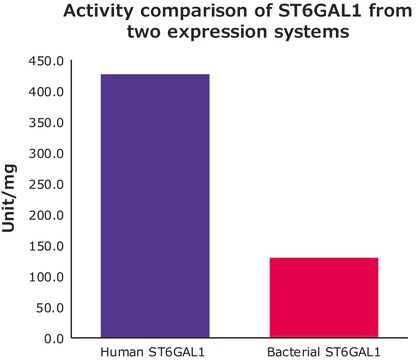
recombinant, expressed in E. coli BL21, ≥5 units/mg protein
Synonyme(s) :
β-Galactoside α-2,6-sialyltransferase, CMP-N-Acetylneuraminate:β-D-galactosyl-1,4-N-acetyl-β-D-glucosamine α-2,6-N-acetylneuraminyltransferase
About This Item
Produits recommandés
Produit recombinant
expressed in E. coli BL21
Niveau de qualité
Forme
lyophilized powder
Activité spécifique
≥5 units/mg protein
Poids mol.
56.8 kDa
Conditions d'expédition
dry ice
Température de stockage
−20°C
Description générale
Application
Actions biochimiques/physiologiques
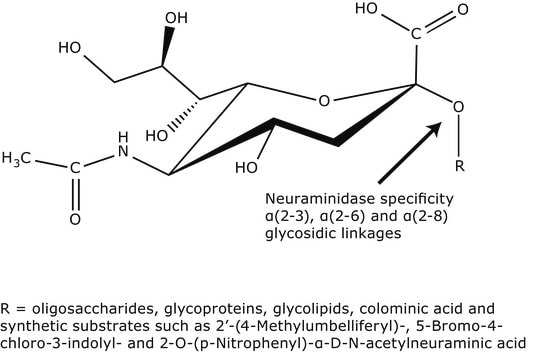
Définition de l'unité
Forme physique
Remarque sur l'analyse
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique