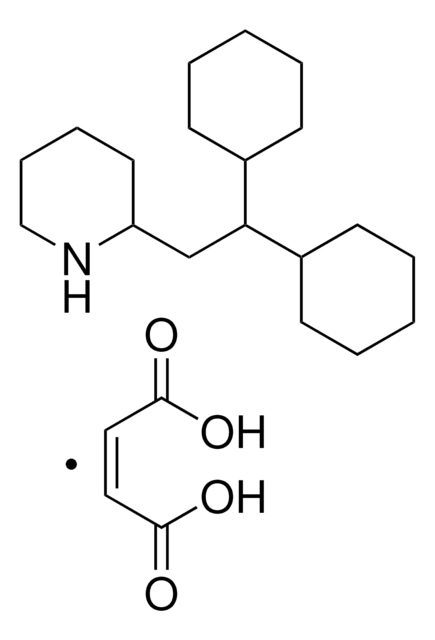
PZ0160
UK-5099
≥98% (HPLC), powder, mitochondrial pyruvate carrier inhibitor
Synonyme(s) :
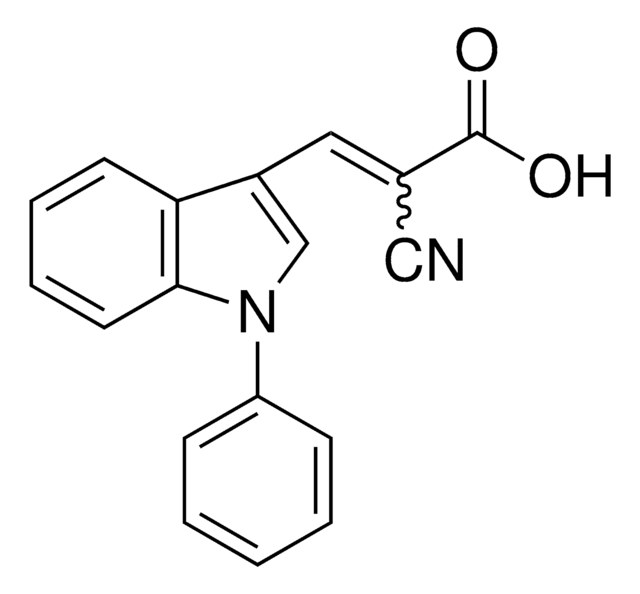
2-Cyano-3-(1-phenyl-1H-indol-3-yl)-2-propenoic acid, PF-1005023
About This Item
Produits recommandés
Nom du produit
UK-5099, ≥98% (HPLC)
Niveau de qualité
Essai
≥98% (HPLC)
Forme
powder
Couleur
yellow to tan
Solubilité
DMSO: >20 mg/mL
Température de stockage
2-8°C
Chaîne SMILES
OC(=O)\C(=C\c1cn(-c2ccccc2)c3ccccc13)C#N
InChI
1S/C18H12N2O2/c19-11-13(18(21)22)10-14-12-20(15-6-2-1-3-7-15)17-9-5-4-8-16(14)17/h1-10,12H,(H,21,22)/b13-10+
Clé InChI
BIZNHCWFGNKBBZ-JLHYYAGUSA-N
Application
- as a mitochondrial pyruvate blocker to reduce pyruvate transportation into mitochondria in Roswell park memorial institute (RPMI) 1640 medium for prostatic cancer cell line culture
- in dimethyl sulfoxide (DMSO) stock, to study the effect of inhibiting pyruvate transport into mitochondria on pro-inflammatory responses in lipopolysaccharide activated macrophages
- in topical treatment in order to study its effect on hair cycle induction in experimental mice
Actions biochimiques/physiologiques
Caractéristiques et avantages
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique