N9150
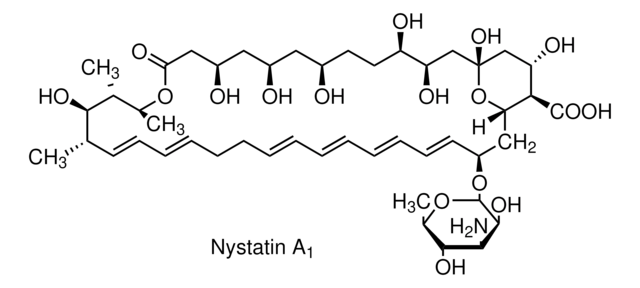
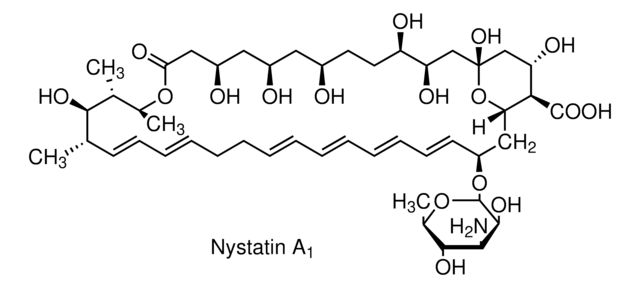
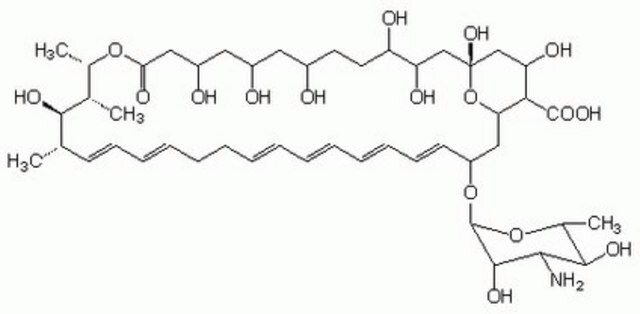
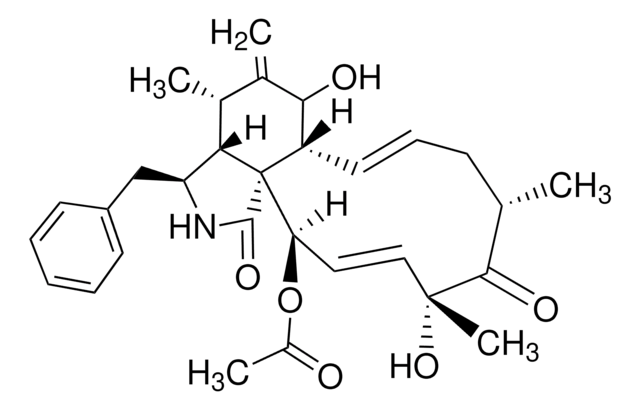
Nystatin
ready made solution, suitable for cell culture
Synonyme(s) :
Nystatin, Fungicidin, Mycostatin
About This Item
Produits recommandés
product name
Nystatin Ready made solution, suitable for cell culture
Source biologique
Streptomyces noursei
Forme
solution
Technique(s)
cell culture | mammalian: suitable
Conditions d'expédition
dry ice
Température de stockage
−20°C
Chaîne SMILES
O[C@@H]([C@H](C)[C@H](C)O1)[C@@H](C)/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@]3([H])[C@H](C(O)=O)[C@@H](O)C[C@](O)(O3)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)C[C@@H](O)CC1=O
InChI
1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28+,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1
Clé InChI
VQOXZBDYSJBXMA-QEKUPDCNSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Actions biochimiques/physiologiques
Antimicrobial spectrum: Nystatin acts against fungi, yeasts and molds.
Caractéristiques et avantages
- Nystatin ready made solution is a fully solubilized formulation of Nystatin with potency of 8000-13000 units/ml according to USP standard.
- Nystatin ready made solution is 0.2μm filtered, endotoxin.
- The suggested working concentration of Nystatin ready made solution is 30 units/ml.
- The ready made solution is light sensitive. It is recommended to store the solution in the dark.
- It is recommended to avoid freeze thaw cycles of this product. Therefore, it is recommended to aliquot and store at -20°C.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique