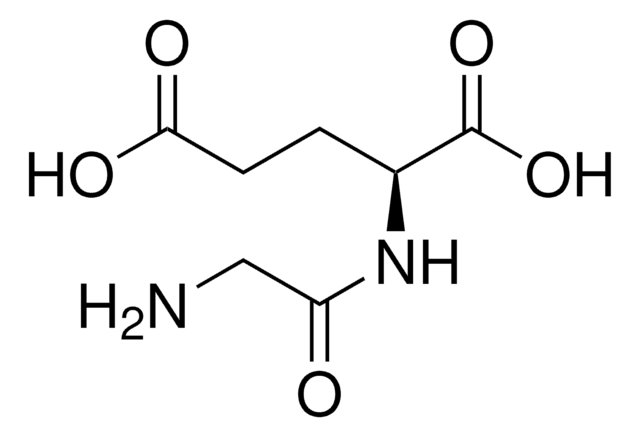
A0912
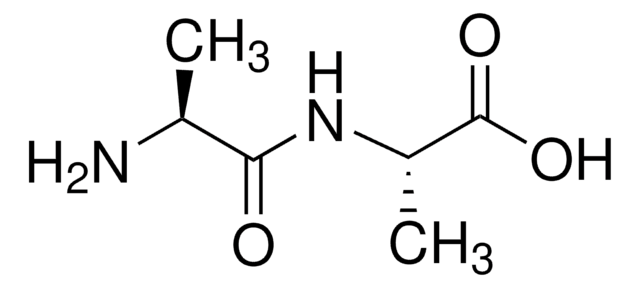
D-Ala-D-Ala
≥99%
Synonyme(s) :
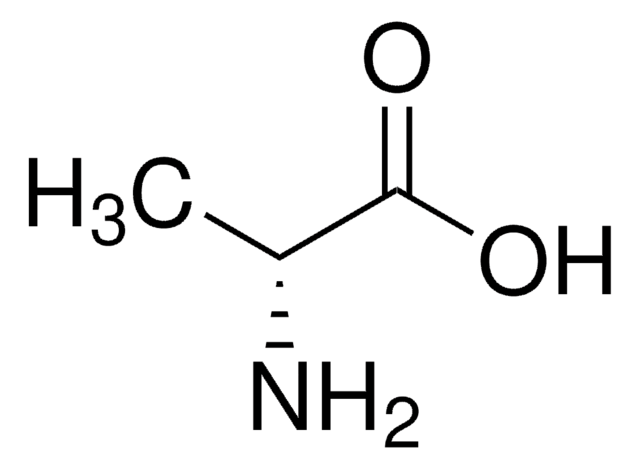
D-alanyl-D-Alanine
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
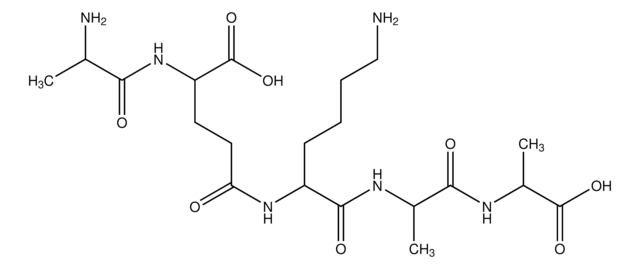
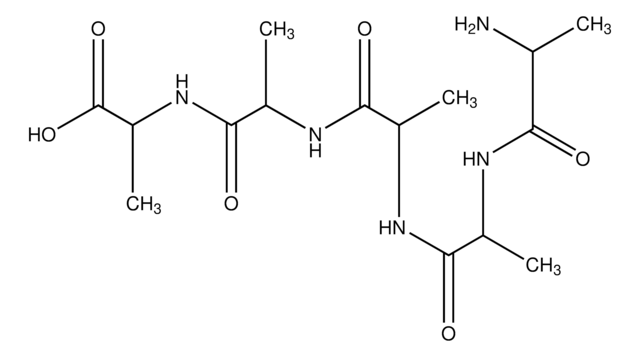
C6H12N2O3
Numéro CAS:
Poids moléculaire :
160.17
Numéro MDL:
Code UNSPSC :
12352202
ID de substance PubChem :
Nomenclature NACRES :
NA.26
Produits recommandés
Nom du produit
D-Ala-D-Ala,
Essai
≥99%
Niveau de qualité
Forme
powder
Couleur
white to off-white
Température de stockage
−20°C
Chaîne SMILES
C[C@@H](N)C(=O)N[C@H](C)C(O)=O
InChI
1S/C6H12N2O3/c1-3(7)5(9)8-4(2)6(10)11/h3-4H,7H2,1-2H3,(H,8,9)(H,10,11)/t3-,4-/m1/s1
Clé InChI
DEFJQIDDEAULHB-QWWZWVQMSA-N
Application
- Binding Mode-Based Physicochemical Screening Method Using d-Ala-d-Ala Silica Gel and Chemical Modification Approach to Facilitate Discovery of New Macrolactams, Banglactams A and B, from Nonomuraea bangladeshensis K18-0086.: Describes a novel screening method employing D-Ala-D-Ala silica gel to discover new macrolactams with potential antibacterial properties. This technique aids in identifying compounds that inhibit bacterial cell wall synthesis (Kimishima et al., 2024).
Actions biochimiques/physiologiques
D-Ala-D-Ala is found in the stem termini of peptidoglycan side-chain pentapeptide found in the cell walls of gram positive bacteria. The D-ala-d-ala stem termini is the site of interaction of glycopeptide antibiotics such as vancomycin and teicoplanin. D-ala-D-ala is a substrate used to study kinetics of UDPMurNAc-tripeptide D-alanyl-D-alanine-adding (ligase) enzyme.
D-Ala-D-Ala, a terminus moiety of bacterial peptidoglycans, is used for affinity chromatography and binding mechanism studies of antibiotics such as teicoplanin, ristocetin, vancomycin.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Yoshiaki Kitamura et al.
Acta crystallographica. Section D, Biological crystallography, 65(Pt 10), 1098-1106 (2009-09-23)
D-Alanine-D-alanine ligase (Ddl) is one of the key enzymes in peptidoglycan biosynthesis and is an important target for drug discovery. The enzyme catalyzes the condensation of two D-Ala molecules using ATP to produce D-Ala-D-Ala, which is the terminal peptide of
Maulik N Thaker et al.
Antimicrobial agents and chemotherapy, 59(3), 1405-1410 (2014-12-17)
Vancomycin-resistant enterococci (VRE) are notorious clinical pathogens restricting the use of glycopeptide antibiotics in the clinic setting. Routine surveillance to detect VRE isolated from patients relies on PCR bioassays and chromogenic agar-based test methods. In recent years, we and others
Gareth A Prosser et al.
Antimicrobial agents and chemotherapy, 60(10), 6091-6099 (2016-08-03)
The increasing global prevalence of drug resistance among many leading human pathogens necessitates both the development of antibiotics with novel mechanisms of action and a better understanding of the physiological activities of preexisting clinically effective drugs. Inhibition of peptidoglycan (PG)
I Tytgat et al.
Current medicinal chemistry, 16(20), 2566-2580 (2009-07-16)
DD-ligases catalyze the synthesis of the D-Ala-D-Ala and D-Ala-D-Ser dipeptides or the D Ala-D-Lac depsipeptide in an early step of peptidoglycan synthesis. Their function is essential for bacterial growth and specific to bacteria, making them attractive targets for the development
Ivona Pavkova et al.
Frontiers in cellular and infection microbiology, 7, 503-503 (2018-01-13)
The DsbA homolog of
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique