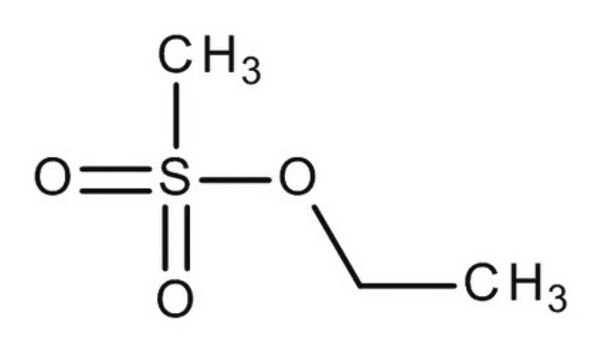
Y0001304
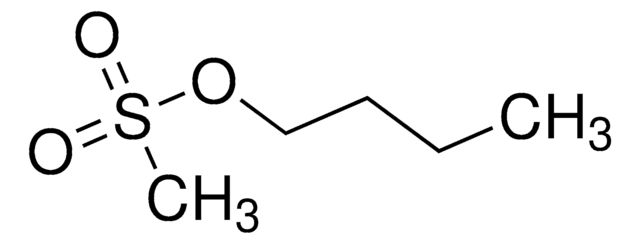
Butyl methanesulfonate
European Pharmacopoeia (EP) Reference Standard
Synonyme(s) :
NSC 36060
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
imatinib
Fabricant/nom de marque
EDQM
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-8°C
InChI
1S/C5H12O3S/c1-3-4-5-8-9(2,6)7/h3-5H2,1-2H3
Clé InChI
LFLBHTZRLVHUQC-UHFFFAOYSA-N
Description générale
Application
Conditionnement
Autres remarques
Produit(s) apparenté(s)
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique