219362
Cathepsin B, Human Liver
Cathepsin B, Human Liver, CAS 9047-22-7, is a purified native cathepsin B from human liver, purified by affinity chromatography. Upregulated in many types of tumors.
Synonyme(s) :
Cathepsin B, Human Liver, cat B, cysteine cathepsin
About This Item
Produits recommandés
Source biologique
human liver
Niveau de qualité
Essai
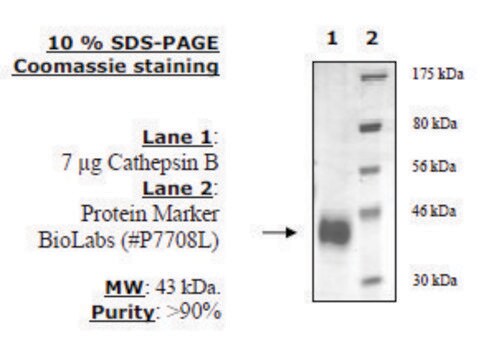
≥95% (SDS-PAGE)
Forme
liquid
Activité spécifique
≥10 units/mg protein
Produit purifié par
affinity chromatography
Fabricant/nom de marque
Calbiochem®
Conditions de stockage
OK to freeze
avoid repeated freeze/thaw cycles
Technique(s)
activity assay: suitable
Adéquation
suitable for molecular biology
Application(s)
life science and biopharma
Conditions d'expédition
wet ice
Température de stockage
−70°C
Informations sur le gène
human ... CTSB(1508)
Description générale
Native cathepsin B from human liver, purified by affinity chromatography and HPLC. The most investigated enzyme of all lysosomal cysteine proteases. Cathepsin B belongs to the papain-like family of cysteine proteases and is produced as a preproenzyme. It is a bilobal protein, and its catalytic site is situated at the interface between the two lobes.
Application
- Diagnostics: as a potent and independent prognostic marker for endometrial cancer, pancreatic adenocarcinoma, and inflammatory disease.
- Drug development: during the neovascularization process and as a potent therapeutic target for various pathologies, cancer progression, and osteoarthritis in humans.
- Pharmacology: for increasing the therapeutic index of doxorubicin by incorporating the cathepsin B cleavable spacer Phe-Lys-4-aminobenzyloxycarbonyl into an albumin-binding prodrug.
- Molecular biology: in cathepsin B activity assay.
Actions biochimiques/physiologiques
Avertissement
Définition de l'unité
Forme physique
Notes préparatoires
Reconstitution
Autres remarques
Kostoulas, G., et al. 1999. FEBS Lett.455, 286.
Strojnik, T., et al. 1999. Clin. Cancer Res.5, 559.
Maquire, T.M., et al. 1998. Int. J. Biol. Markers13, 139.
Berquim, I.M., and Sloane, B.F. 1996. Adv. Exp. Med. Biol.389, 281.
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique