1.46429
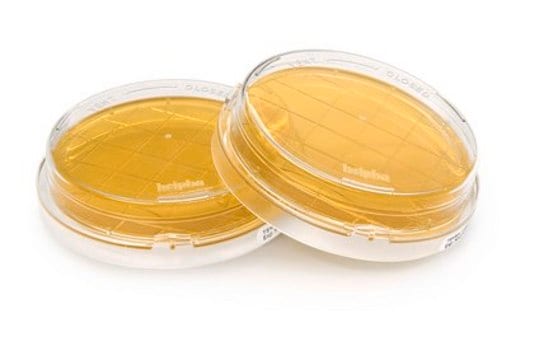
Gélose tryptone-soja - Flacons et tuyaux prêts à l'emploi
tube capacity 25 mL, tube filling volume 18 mL, closure type, blue screw cap with septum, box of 20 or 100 tubes
Synonyme(s) :
CASO Agar, Casein Soybean Digest Agar, TSA, Tryptic Soy Agar, Tryptone Soya Agar
About This Item
Produits recommandés
Agence
EP 2.6.13
JP 4.05
USP 62
Niveau de qualité
Description
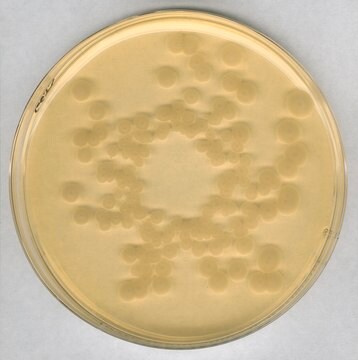
For microbiological examination of non-sterile products
Stérilité
sterile
Forme
liquid (ready-to-use)
Durée de conservation
12 mo.
Caractéristiques
closure type blue screw cap with septum
ready-to-use
Conditionnement
box of 20 or 100 tubes
single packed of (18 ml in 25 ml tube with blue cap (20 or 100 tubes per box))
Technique(s)
direct inoculation: suitable
sterile filtration: suitable
Capacité du tube
25 mL
tube filling volume
18 mL
Couleur
clear yellowish
pH
7.3
Application(s)
bioburden testing
cosmetics
food and beverages
pharmaceutical
Température de stockage
2-25°C
Catégories apparentées
Description générale
Application
Caractéristiques et avantages
- Our ready-to-use media provide the highest level of quality and testing confidence. They have been formulated and tested to meet the pharmacopoeia requirements.
- Sterility testing media and rinse solutions are manufactured in an ISO 9001, environmentally controlled production center. Each lot undergoes a stringent quality control (QC) procedure, including pH, sterility, and growth promotion tests.
- Our manufacturing approach ensures the highest level of clarity for our media and rinsing fluids, therefore improving accuracy, and significantly reducing the risk of incorrect interpretation and false results.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique