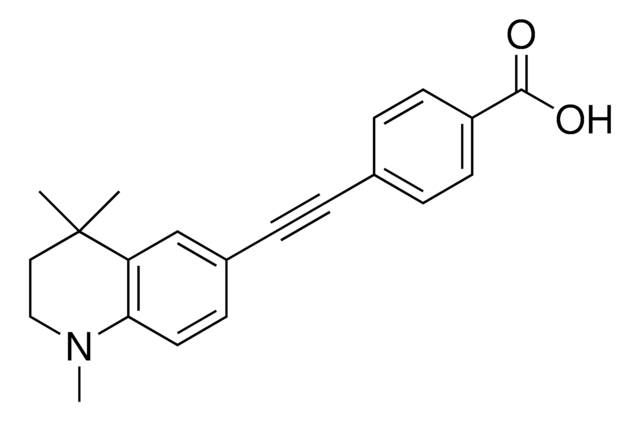
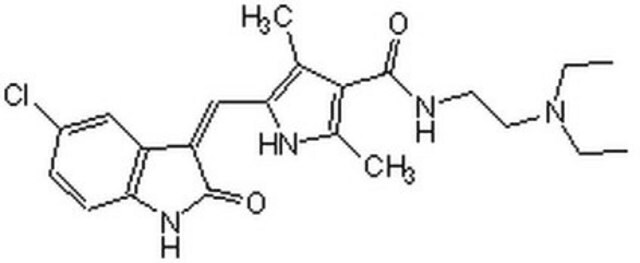
14-555-M
Alk Protein, active, 10 µg
Active, N-terminal His6-tagged, recombinant human ALK, residues 1058-end, for use in Kinase Assays.
Synonyme(s) :
Anaplastic lymphoma kinase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352202
eCl@ss :
32160405
Nomenclature NACRES :
NA.32
Produits recommandés
Source biologique
human
Niveau de qualité
Produit recombinant
expressed in baculovirus infected Sf21 cells
Poids mol.
Mw 63.8 kDa
Fabricant/nom de marque
Upstate®
Technique(s)
activity assay: suitable (kinase)
Numéro d'accès NCBI
Numéro d'accès UniProt
Description générale
Research area: CELL SIGNALING
Product Source: expressed by baculovirus in Sf21 cells
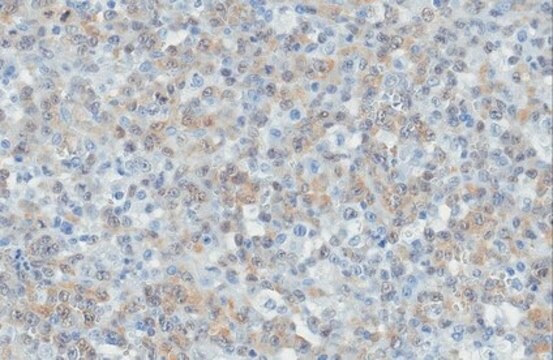
The anaplastic lymphoma kinase (ALK) is a member of the insulin receptor superfamily of receptor tyrosine kinases. The ALK gene is located on human chromosome 2p23 and shares a high similarity with the leukocyte tyrosine kinase (Ltk). ALK is composed of three domains: an extracellular domain responsible for ligand binding, a transmembrane domain that spans the cell membrane, and a cytoplasmic domain housing the tyrosine kinase activity. The cytoplasmic tyrosine kinase domain of ALK plays a significant role in disease pathologies associated with the gene. Within the structure, there are two short conserved β-strands (β7-β8) housing majority of the catalytic residues. Full-length ALK is specifically expressed in the developing central and peripheral nervous systems during embryogenesis and is associated with the balance of cell proliferation and differentiation.
Product Source: expressed by baculovirus in Sf21 cells
The anaplastic lymphoma kinase (ALK) is a member of the insulin receptor superfamily of receptor tyrosine kinases. The ALK gene is located on human chromosome 2p23 and shares a high similarity with the leukocyte tyrosine kinase (Ltk). ALK is composed of three domains: an extracellular domain responsible for ligand binding, a transmembrane domain that spans the cell membrane, and a cytoplasmic domain housing the tyrosine kinase activity. The cytoplasmic tyrosine kinase domain of ALK plays a significant role in disease pathologies associated with the gene. Within the structure, there are two short conserved β-strands (β7-β8) housing majority of the catalytic residues. Full-length ALK is specifically expressed in the developing central and peripheral nervous systems during embryogenesis and is associated with the balance of cell proliferation and differentiation.
Application
Research Category
Inflammation & Immunology
Inflammation & Immunology
Actions biochimiques/physiologiques
Protein Target: ALK
Target Sub-Family: TK Anaplastic lymphoma kinase (ALK) plays a crucial role in the development of neurons. Binding of ligand to its extracellular domain triggers the activation of the tyrosine kinase that in turn activates the mitogen-activated protein kinase (MAPK) pathway. ALK fusion proteins induce the activation of diverse pathways including Phospholipase C gamma (PLC-γ) and Ras/Extracellular Signal-Regulated Kinase 1/2 (Ras/ERK1/2), which contribute to cell proliferation, while the Janus Kinase/signal transducer and activator of transcription (JAK/STAT) pathway and Phosphoinositide 3-kinase/Protein Kinase B (PI3K/Akt) pathways facilitate cell survival. Notably, ALK has been found to be overexpressed in various human tumors and cell lines such as lymphomas, neuroblastoma, and non-small-cell lung cancer.
Target Sub-Family: TK Anaplastic lymphoma kinase (ALK) plays a crucial role in the development of neurons. Binding of ligand to its extracellular domain triggers the activation of the tyrosine kinase that in turn activates the mitogen-activated protein kinase (MAPK) pathway. ALK fusion proteins induce the activation of diverse pathways including Phospholipase C gamma (PLC-γ) and Ras/Extracellular Signal-Regulated Kinase 1/2 (Ras/ERK1/2), which contribute to cell proliferation, while the Janus Kinase/signal transducer and activator of transcription (JAK/STAT) pathway and Phosphoinositide 3-kinase/Protein Kinase B (PI3K/Akt) pathways facilitate cell survival. Notably, ALK has been found to be overexpressed in various human tumors and cell lines such as lymphomas, neuroblastoma, and non-small-cell lung cancer.
Qualité
routinely evaluated by phosphorylation of IGF-1Rtide
Forme physique
Ni2+/NTA-agarose
Stockage et stabilité
6 months at -70°C from date of shipment
Autres remarques
For Specific Activity data, refer to the Certificate of Analysis for individual lots of this enzyme.
Informations légales
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Clause de non-responsabilité
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Expression of anaplastic lymphoma kinase in non-Hodgkin's lymphomas and other malignant neoplasms. Biological, diagnostic, and clinical implications.
Wasik, Mariusz A
American Journal of Clinical Pathology, 118 Suppl, S81-S92 (2002)
K Pulford et al.
Cellular and molecular life sciences : CMLS, 61(23), 2939-2953 (2004-12-08)
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase, the normal role of which remains to be completely elucidated. Although work carried out in mammals suggests a function in neural development, results from studies in Drosophila indicate an additional role
Anaplastic lymphoma kinase proteins in growth control and cancer.
Pulford, K, et al.
Journal of Cellular Physiology, 199, 330-358 (2004)
ALK a novel lymphoma-associated tumor antigen for vaccination strategies.
Passoni, Lorena and Gambacorti-Passerini, Carlo
Leukemia & Lymphoma, 44, 1675-1681 (2003)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique