M54104
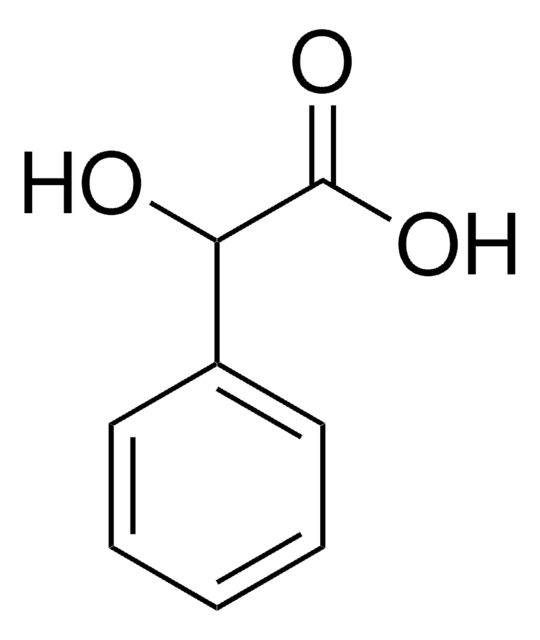
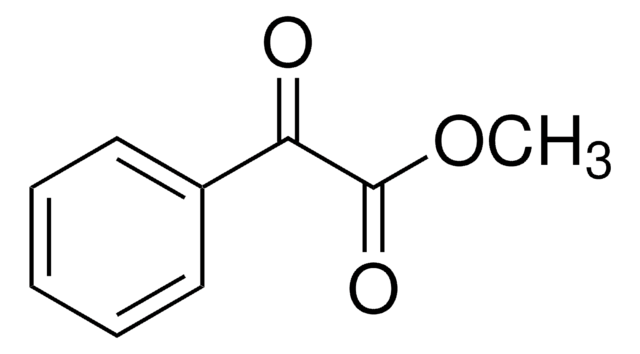
Methyl DL-mandelate
97%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
C6H5CH(OH)COOCH3
Numéro CAS:
Poids moléculaire :
166.17
Beilstein:
2047558
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
97%
pb
135 °C/12 mmHg (lit.)
Pf
54-56 °C (lit.)
Chaîne SMILES
COC(=O)C(O)c1ccccc1
InChI
1S/C9H10O3/c1-12-9(11)8(10)7-5-3-2-4-6-7/h2-6,8,10H,1H3
Clé InChI
ITATYELQCJRCCK-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
235.4 °F - closed cup
Point d'éclair (°C)
113 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Fei Guo et al.
Applied microbiology and biotechnology, 97(8), 3355-3362 (2012-11-28)
Despite directed evolution being a practical and efficient method of improving the properties of enzymes, a trade-off between the targeted property and other essential properties often exists which hinders the efficiency of directed evolution. In our previous work, mutant CVH
Y Chiang et al.
The Journal of organic chemistry, 65(4), 1175-1180 (2000-05-18)
Flash photolysis of methyl phenyldiazoacetate in aqueous solution produced phenylcarbomethoxycarbene, whose hydration generated a short-lived transient species that was identified as the enol isomer of methyl mandelate. This assignment is supported by the shape of the rate profile for decay
Jie-Hua Shi et al.
Journal of molecular modeling, 18(2), 803-813 (2011-05-20)
Host-guest interactions of permethylated β-cyclodextrin (PM-β-CD) with methyl mandelate enantiomers ((R/S)-MMA) were simulated using semiempirical PM3 and ONIOM (B3LYP/6-31G(d):PM3) method. The chiral recognition mechanism of (R/S)-MMA enantiomers on PM-β-CD was investigated. The binding energies for all orientations considered in this
M Y Nie et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 17(10), 1183-1187 (2002-05-07)
Enantiomer separation of mandelates and their analogs, which are important intermediates in asymmetric synthetic and pharmaceutical chemistry, was investigated by capillary gas chromatography using different cyclodextrin derivative chiral stationary phases (CD CSPs). The used cyclodextrin derivatives included permethylated beta-CD (PMBCD)
Han-Ning Wei et al.
Applied biochemistry and biotechnology, 149(1), 79-88 (2008-03-20)
Microorganisms producing lipase were isolated from soil and sewage samples and screened for enantioselective resolution of (R,S)-methyl mandelate to (R)-mandelic acid. A strain designated as GXU56 was obtained and identified as Burkholderia sp. Preparing immobilized GXU56 lipase by simple adsorption
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique