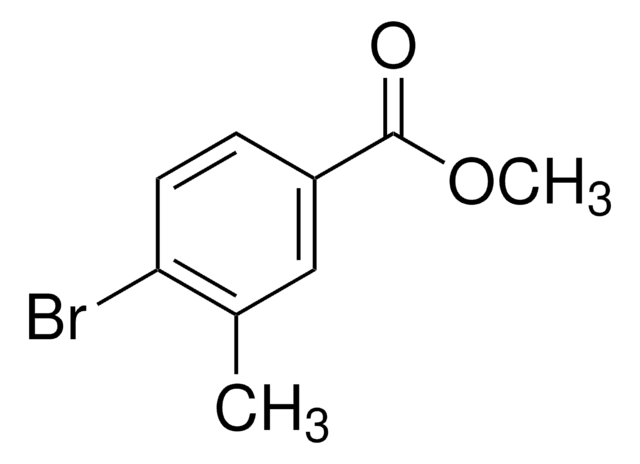
L511188
(S)-2-(2,3-Bis(dicyclohexylamino)cyclopropenimine)-3-phenylpropan-1-ol hydrochloride
AldrichCPR
Synonyme(s) :
(βS)-β-[[2,3-bis(dicyclohexylamino)-2-cyclopropen-1-ylidene]amino]-benzenepropanol hydrochloride (1:1), Dicyclohexyl cyclopropenimine, Lambert Cyclopropenimine Catalyst
About This Item
Produits recommandés
Chaîne SMILES
Cl.OC[C@H](Cc1ccccc1)\N=C2\C(N(C3CCCCC3)C4CCCCC4)=C2N(C5CCCCC5)C6CCCCC6
InChI
1S/C36H55N3O.ClH/c40-27-29(26-28-16-6-1-7-17-28)37-34-35(38(30-18-8-2-9-19-30)31-20-10-3-11-21-31)36(34)39(32-22-12-4-13-23-32)33-24-14-5-15-25-33;/h1,6-7,16-17,29-33,40H,2-5,8-15,18-27H2;1H/t29-;/m0./s1
Clé InChI
RTCSAEOYHXMTHG-JMAPEOGHSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products.
Novel Brønsted bases provide potent yet tunable basicity to the acidity of a given substrate, are trivial to prepare, and offer unique opportunities for asymmetric transition state organization. The high basicity should allow them to catalyze a wide range of reactions (i.e. chiral pharmaceutical ingredients) and could lead to easier and faster syntheses of novel chiral compounds for drug discovery and other applications.
2,3-bis(dialkylamino)cyclopropenimines serve as a highly effective platform for chiral Brønsted base catalysis. Chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. Catalysis with chiral cyclopropenimines should be amenable to relatively large-scale applications under mild reaction conditions.

Autres remarques
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique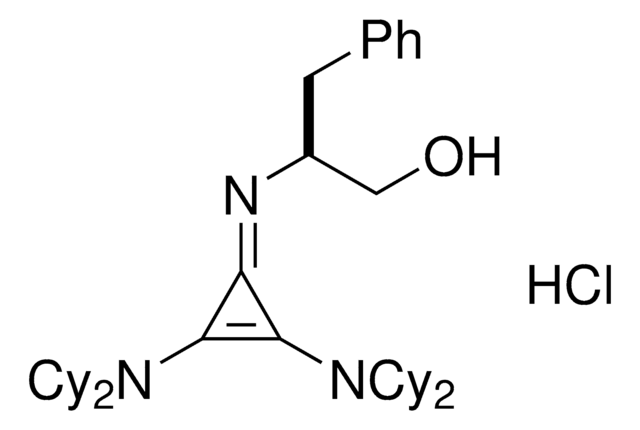

![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)