763896
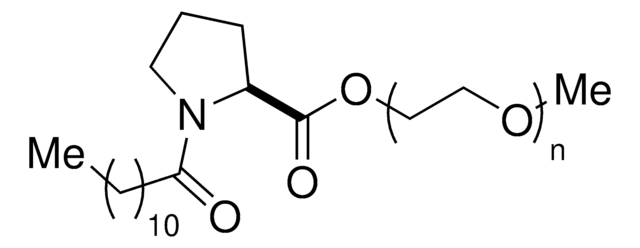
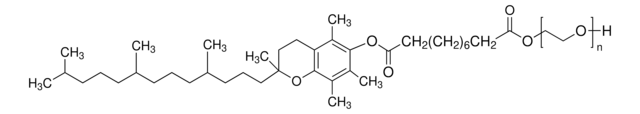
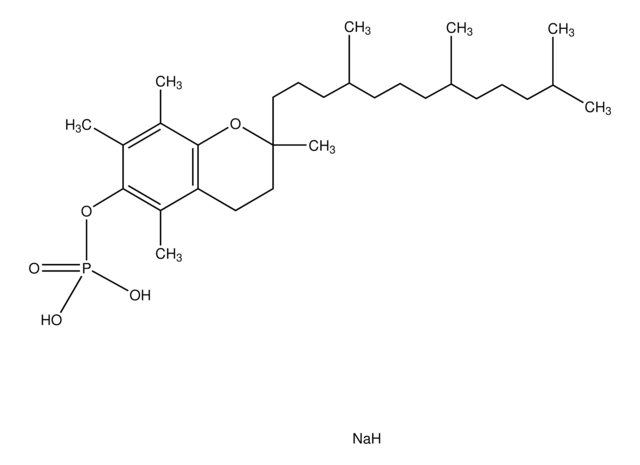
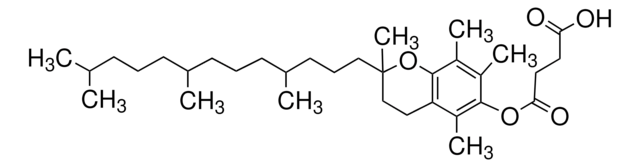
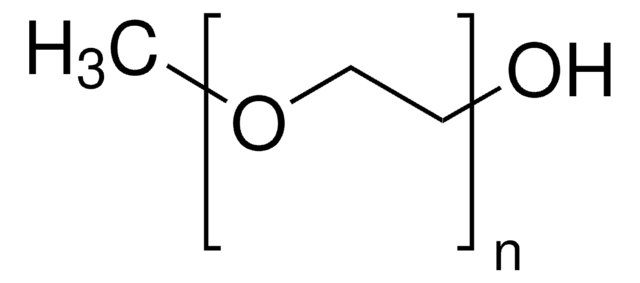
DL-α-Tocopherol methoxypolyethylene glycol succinate
Synonyme(s) :
Polyethylene glycol, TPGS-750-M
About This Item
Produits recommandés
Forme
powder
Niveau de qualité
Pertinence de la réaction
reagent type: catalyst
reaction type: C-H Activation
reagent type: surfactant
Caractéristiques du produit alternatif plus écologique
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Température de transition
Tm 29-34
Autre catégorie plus écologique
, Aligned
Description générale
Application
TPGS-750-M can also be employed in:
- The nucleophilic aromatic substitution reaction of aryl and heteroaryl halides with nitrogen, oxygen, and sulfur nucleophiles under mild conditions.
- The preparation of quinoxaline-2,3 diones from quinoxalinones via C(sp2)-H hydroxylation reaction.
- The intramolecular N-arylation of amines using a copper catalyst.
Autres remarques
From milligrams to kilograms: synthetic chemistry following nature′s lead
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Lipshutz and co-workers have recently developed a second generation technology to their original PTS-enabling surfactant based on the polyoxyethanyl-α-tocopheryl succinate derivative, TPGS-750-M.
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
Protocoles
Buchwald-Hartwig Amination Reaction in Water at Room Temperature using TPGS-750-M
Contenu apparenté
Micellular catalysis has provided the ability to carry out several commonly used transformations used in the synthetic community to be carried out in water.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique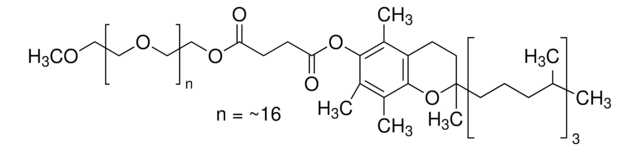
![[1,1′-bis(diphénylphosphino)ferrocène]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)