546682
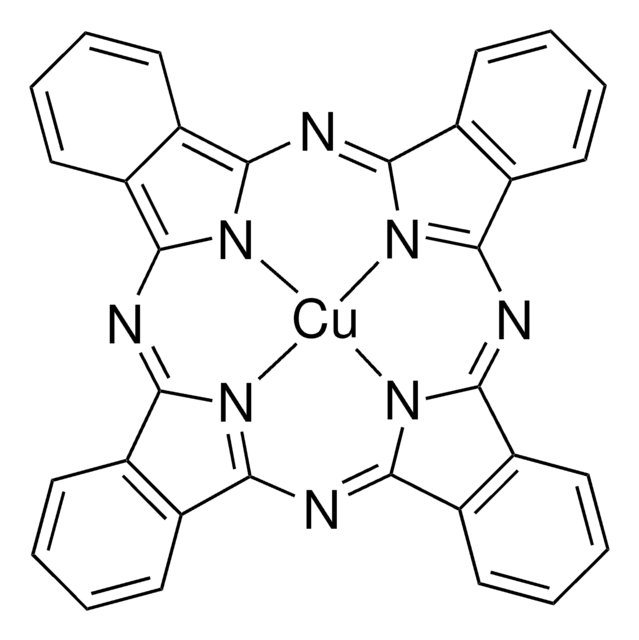
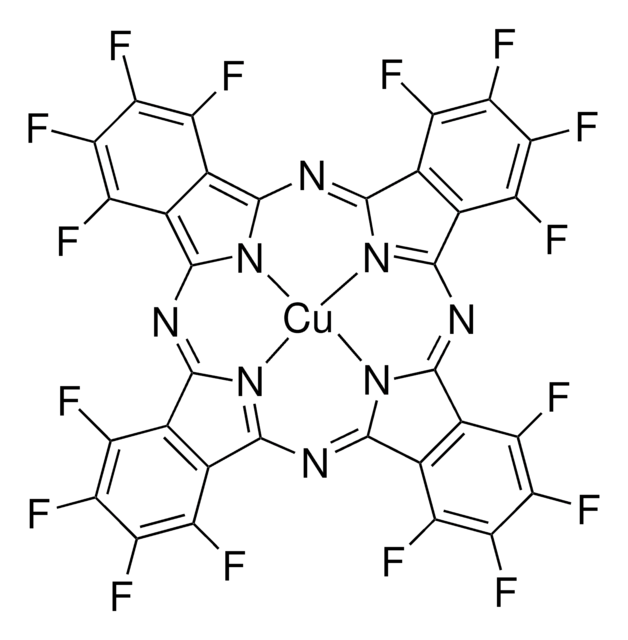
Copper(II) phthalocyanine
Dye content >99 %
Synonyme(s) :
CuPc
About This Item
Produits recommandés
Composition
Dye content, >99%
Niveau de qualité
λmax
678 nm
Performance des dispositifs OLED
ITO/CuPc/NPD/Alq3/C60/Mg:Ag
ITO/CuPc/NPD/Alq3/LiF/Al
ITO/CuPc/NPD/CBP:FIrpic (6%)/BAlq3/LiF/Al
Performance des dispositifs OPV
ITO/CuPc/PTCDA/In
ITO/PEDOT:PSS/CuPc/C60/BCP/Al
Chaîne SMILES
c1ccc2c(c1)C3=NC4=[N@@H]5C(=Nc6n7c(N=C8c9ccccc9C%10=[N@@H]8[Cu]57N3C2=N%10)c%11ccccc6%11)c%12ccccc4%12
InChI
1S/C32H16N8.Cu/c1-2-10-18-17(9-1)25-33-26(18)38-28-21-13-5-6-14-22(21)30(35-28)40-32-24-16-8-7-15-23(24)31(36-32)39-29-20-12-4-3-11-19(20)27(34-29)37-25;/h1-16H;/q-2;+2
Clé InChI
XCJYREBRNVKWGJ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- A facile molecularly engineered copper (II) phthalocyanine as hole transport material for planar perovskite solar cells with enhanced performance and stability: This study introduces a modified copper(II) phthalocyanine that enhances the performance and stability of perovskite solar cells (Yang et al., 2017).
- Dopant-free methoxy substituted copper (II) phthalocyanine for highly efficient and stable perovskite solar cells: Discusses the synthesis and application of a methoxy-substituted copper(II) phthalocyanine, improving the efficiency and stability of perovskite solar cells (Ding et al., 2020).
- Highly efficient dye-sensitized solar cells based on metal-free and copper (II) phthalocyanine bearing 2-phenylphenoxy moiety: Examines novel phthalocyanines for use in dye-sensitized solar cells, focusing on their synthesis and photophysical properties (Ali et al., 2016).
- Operando HERFD-XANES and surface sensitive Delta mu analyses identify the structural evolution of copper (II) phthalocyanine for electroreduction of CO2: This research uses advanced spectroscopic techniques to explore the structural changes in copper(II) phthalocyanine during CO2 electroreduction (Mei et al., 2022).
- New dye sensitized photocatalysts: Copper (II)-phthalocyanine/TiO2 nanocomposite for water remediation: Studies a copper(II) phthalocyanine-TiO2 composite as a photocatalyst for water remediation, showing its effectiveness in degrading pollutants under light irradiation (Albay et al., 2016).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
nwg
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The conductivity of organic semiconductors can be increased, and the barriers to charge-carrier injection from other materials can be reduced, by the use of highly reducing or oxidizing species to n- or p-dope, respectively, the semiconductor.
Professor Shinar (Iowa State University, USA) summarizes the developments of a variety of sensor configurations based on organic and hybrid electronics, as low-cost, disposable, non-invasive, wearable bioelectronics for healthcare.
While dye sensitization as the basis for color photography has been accepted for a very long time,1 attempts to use this principle for the conversion of solar light to electricity generally had resulted only in very low photocurrents, below 100 nA/cm2.2
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique