493937
3-Methyl-2-oxindole
96%
Synonyme(s) :
3-Methyloxindole
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C9H9NO
Numéro CAS:
Poids moléculaire :
147.17
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
96%
Forme
solid
Pf
117-121 °C (lit.)
Chaîne SMILES
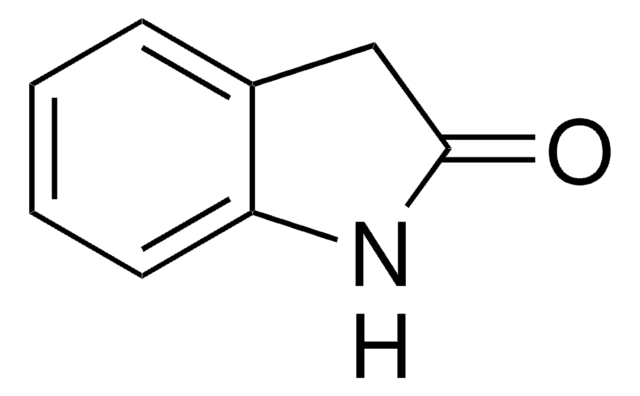
CC1C(=O)Nc2ccccc12
InChI
1S/C9H9NO/c1-6-7-4-2-3-5-8(7)10-9(6)11/h2-6H,1H3,(H,10,11)
Clé InChI
BBZCPUCZKLTAJQ-UHFFFAOYSA-N
Catégories apparentées
Description générale
3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products.
Application
3-Methyl-2-oxindole may be used in the preparation of 3-hydroxy-3-methyl-2-oxindole.
- Reactant for enantioselective α-amination reactions
- Reactant for aldol reaction with glyoxal derivatives
- Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes
- Reactant for O-acetylation reactions
- Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-carbinol, and 3-methylindole in goats.
M J Potchoiba et al.
American journal of veterinary research, 43(8), 1418-1423 (1982-08-01)
Ying Jin et al.
Chirality, 26(12), 801-805 (2014-07-22)
A series of cinchona alkaloid derivatives were used to catalyze the asymmetric anti-Mannich-type reaction of 3-methyl-2-oxindole with N-tosyl aryl aldimines. The resulting anti-3,3-disubstituted 2-oxindole products were obtained in good yields (up to 92%) with high diastereo- and enantioselectivities (anti/syn up
J Thornton-Manning et al.
The Journal of pharmacology and experimental therapeutics, 276(1), 21-29 (1996-01-01)
The toxicity of 3-methylindole (3 MI), a selective pneumotoxin, is dependent upon cytochrome P450-mediated bioactivation 3. Using vaccinia-expressed P450 enzymes, the metabolites of radiolabeled 3 MI produced by 14 individual P450s were identified and quantified by high performance liquid chromatography.
Jaroslav Matal et al.
Neuro endocrinology letters, 30 Suppl 1, 36-40 (2009-12-23)
To study the contribution of individual purified porcine CYP1A2, 2E1 and 2A19 enzymes to the biotransformation of skatole. Individual porcine and human enzymes (CYP1A2, 2E1 or 2A6/19) were used to study their potential involvement in skatole metabolism. Furthermore, the inhibition
Takuma Sakurai et al.
Microorganisms, 7(9) (2019-09-14)
Recent studies have shown that metabolites produced by microbes can be considered as mediators of host-microbial interactions. In this study, we examined the production of tryptophan metabolites by Bifidobacterium strains found in the gastrointestinal tracts of humans and other animals.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique