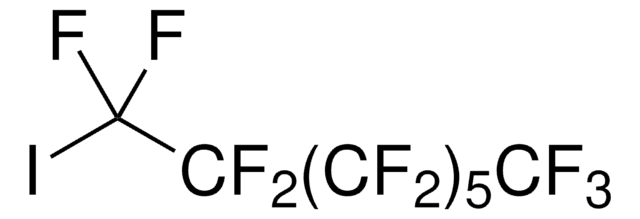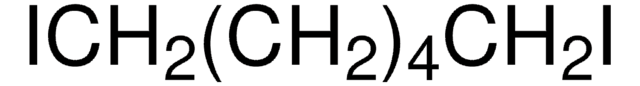473863
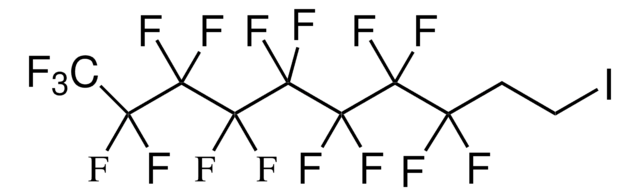
Octafluoro-1,4-diiodobutane
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
I(CF2)4I
Numéro CAS:
Poids moléculaire :
453.84
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
98%
Indice de réfraction
n20/D 1.429 (lit.)
pb
150 °C (lit.)
Pf
−9 °C (lit.)
Densité
2.474 g/mL at 25 °C (lit.)
Groupe fonctionnel
fluoro
iodo
Chaîne SMILES
FC(F)(I)C(F)(F)C(F)(F)C(F)(F)I
InChI
1S/C4F8I2/c5-1(6,3(9,10)13)2(7,8)4(11,12)14
Clé InChI
JILAKKYYZPDQBE-UHFFFAOYSA-N
Catégories apparentées
Description générale
Octafluoro-1,4-diiodobutane (ofib, C4F8I2) is also referred to as 1,1,2,2,3,3,4,4-octafluoro-1,4-diiodobutane. It acts as a halogen bond donor compound and its cocrystallization with methyldiphenylphosphine oxide (mdppo) has been investigated. In combination with a calixcrown compound ofib forms a ′binary host′ system, which is employed for the isolation of cesium iodide from aqueous to fluorous phase.
Application
Octafluoro-1,4-diiodobutane may be used as a guest molecule to prepare a chiral bidentate inclusion complex with pyridyl moieties of a pyridoallenoacetylenic host. It may be employed in the synthesis of three-component supramolecular complexes.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Silvia Castro-Fernández et al.
Organic letters, 16(4), 1136-1139 (2014-02-12)
A chiral bidentate inclusion complex has been formed by halogen-bond interaction between the pyridyl moieties of a pyridoallenoacetylenic host and octafluorodiiodobutane. X-ray crystallography showed that the guest adopts a chiral conformation inside the molecular channels formed by stacking of the
Switching between halogen-and hydrogen-bonding in stoichiometric variations of a cocrystal of a phosphine oxide.
Oh SY, et al.
CrystEngComm, 14(19), 6110-6114 (2012)
Dipyridinocalixcrown/diiodoperfluorocarbon binary host systems for CsI: structural studies and fluorous phase extraction of caesium.
Gattuso G, et al.
Tetrahedron, 63(23), 4951-4958 (2007)
Metric engineering of supramolecular Borromean rings.
Liantonio R, et al.
Chemical Communications (Cambridge, England), 17, 1819-1821 (2006)
Liang Lu et al.
Journal of applied toxicology : JAT, 39(7), 945-954 (2019-03-06)
Fluorinated diiodine alkanes (FDIAs), important industrial intermediates in the synthesis of various perfluorinated compounds, which are distributed widely in wildlife and humans. Recent studies showed that FDIAs had in vitro estrogenic effects. However, to date, little information is available regarding
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![N-[4-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl) benzyloxycarbonyloxy]succinimide ≥97.0% (NMR)](/deepweb/assets/sigmaaldrich/product/structures/232/278/7cb408c4-7b05-4cf3-8e6d-6886bb873b9c/640/7cb408c4-7b05-4cf3-8e6d-6886bb873b9c.png)