151874
Sulfoxyde de diméthyle-d6, Diméthylsulfoxyde-d6
99.9 atom % D
Synonyme(s) :
(Sulfoxyde de méthyle)-d6, (Méthylsulfoxyde)-d6, DMSO-d6, Hexadeutérodiméthyl sulfoxyde, Hexadeutérodiméthylsulfoxyde
About This Item
Produits recommandés
Pression de vapeur
0.42 mmHg ( 20 °C)
Niveau de qualité
Pureté isotopique
99.9 atom % D
Pureté
99% (CP)
Forme
liquid
Température d'inflammation spontanée
573 °F
Limite d'explosivité
42 %
Technique(s)
NMR: suitable
Impuretés
≤0.0250% water
water
Indice de réfraction
n20/D 1.476 (lit.)
Point d'ébullition
189 °C (lit.)
Pf
20.2 °C (lit.)
Densité
1.190 g/mL at 25 °C (lit.)
Changement de masse
M+6
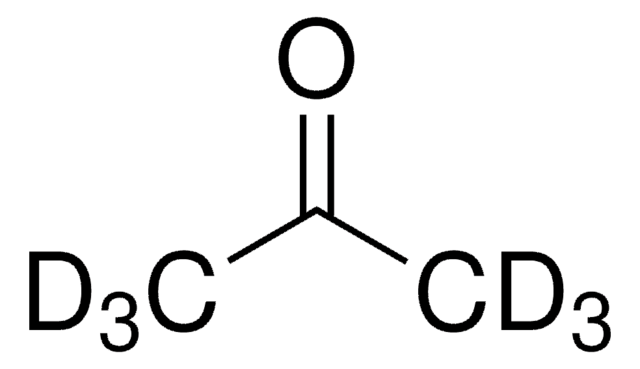
Chaîne SMILES
[2H]C([2H])([2H])S(=O)C([2H])([2H])[2H]
InChI
1S/C2H6OS/c1-4(2)3/h1-2H3/i1D3,2D3
Clé InChI
IAZDPXIOMUYVGZ-WFGJKAKNSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Investigation of the Spatial Structure of Flufenamic Acid in Supercritical Carbon Dioxide Media via 2D NOESY.: This research explores the molecular interactions of flufenamic acid in supercritical CO2, highlighting analytical techniques using Dimethyl sulfoxide-d₆ as a solvent to enhance spectral readings (Khodov et al., 2023).
- Taguchi Approach for Optimization of a Green Quantitative 1H-NMR Practice for Characterization of Levetiracetam and Brivaracetam in Pharmaceuticals.: Utilizes Dimethyl sulfoxide-d₆ in NMR spectroscopy to refine analytical methods for characterizing pharmaceuticals, aiming for environmental sustainability (Mansour et al., 2022).
- Counterintuitive torsional barriers controlled by hydrogen bonding.: Investigates molecular torsion influenced by hydrogen bonding, with Dimethyl sulfoxide-d₆ employed to study solvent effects, contributing to our understanding of molecular dynamics in analytical chemistry (Barbero et al., 2020).
- Elucidating Interactions between DMSO and Chelate-Based Ionic Liquids.: Examines the interaction dynamics between Dimethyl sulfoxide-d₆ and ionic liquids, offering insights into solvent-solute interactions critical in analytical methodologies (Chen et al., 2015).
- Conformation of an octapeptide fragment (2-9) of kaliocin-1 in DMSO-d6 by 1H NMR and restrained molecular dynamics.: This study uses Dimethyl sulfoxide-d₆ in NMR to elucidate the conformational properties of a peptide, aiding in the understanding of peptide structure under analytical conditions (Sunilkumar et al., 2007).
Produits recommandés
À utiliser avec
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
190.4 °F
Point d'éclair (°C)
88 °C
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Use this reference table to find the coupling values and chemical shifts of our NMR (deuterated) solvents. Melting and boiling points, molecular weight, density, and CAS number are also listed.
Contenu apparenté
NMR spectroscopy elucidates molecular structure and purity via nuclear spin states in a strong magnetic field.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique