SRP6215
Enterokinase human
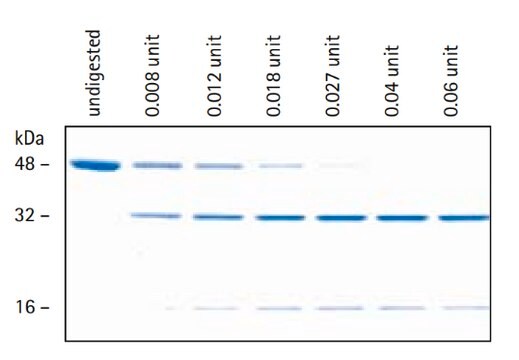
recombinant, expressed in CHO cells, ≥90% (SDS-PAGE)
Synonym(s):
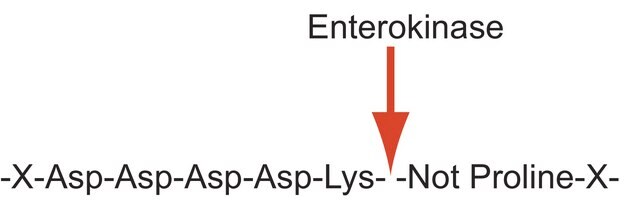
Enteropeptidase, Serine protease 7, transmembrane protease serine 15
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
biological source
human
recombinant
expressed in CHO cells
Assay
≥90% (SDS-PAGE)
form
lyophilized powder
mol wt
97.5 kDa
packaging
pkg of 10 and 50 μg
impurities
<2 EU/μg endotoxin (LAL test)
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... ENTK(5651)
General description
The enzyme enterokinase is a serine protease that is encoded by a 3696 nucleotide cDNA that contains an open reading frame of 3057 nucleotides. The encoded precursor is cleaved to form an active protein that contains a 784 amino acid heavy chain followed by a 235 amino acid light chain that are linked by one or more disulfide bonds. The mRNA is found to be expressed only in small intestine and the protein is found in enterocytes of duodenum and proximal jejunum . The human enterokinase gene is localized to chromosome 21q21.
Application
Enterokinase has been used in the activation of pancreatic enzyme preparation. It has been used for the activation and determination of trypsin activity in pancreatic enzyme secretion.
Biochem/physiol Actions
The enterokinase enzyme is a glycoprotein that catalyzes the conversion of trypsinogen to trypsin, which activates the zymogens pancreatic digestive enzymes. Deficiency of this enzyme causes protein deficiency as zymogens do not get activated. Congenital deficiency of enteropeptidase leads to severe intestinal malabsorption with diarrhea, vomiting, and growth failure.
Physical form
Sterile filtered through a 0.2 micron filter. Lyophilized from 10 mM Sodium Phosphate, pH 7.5 and 1 mM Calcium Chloride.
Reconstitution
Centrifuge the vial prior to opening. Reconstitute in water to a concentration of 0.1-1.0 mg/mL. Do not vortex. This solution can be stored at 2-8°C for up to 1 week. For extended storage, it is recommended to further dilute in a buffer containing a carrier protein (example 0.1% BSA) and store in working aliquots at -20°C to -80°C.
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Expression, purification, and characterization of human enteropeptidase catalytic subunit in Escherichia coli.
Gasparian ME
Protein Expression and Purification, 31, 133-139 (2003)
Purification and sequencing of a trypsin-sensitive cholecystokinin-releasing peptide from rat pancreatic juice. Its homology with pancreatic secretory trypsin inhibitor.
Iwai K
The Journal of Biological Chemistry, 262, 8956-8959 (1987)
Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats.
Iwaki M
Japanese Journal of Pharmacology, 41, 155-162 (1986)
Immunofluorescent localisation of enterokinase in human small intestine.
Hermon-Taylor J
Gut, 18, 259-265 (1977)
Y Kitamoto et al.
Biochemistry, 34(14), 4562-4568 (1995-04-11)
Enterokinase is a serine protease of the duodenal brush border membrane that cleaves trypsinogen and produces active trypsin, thereby leading to the activation of many pancreatic digestive enzymes. Overlapping cDNA clones that encode the complete human enterokinase amino acid sequence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service