D7408
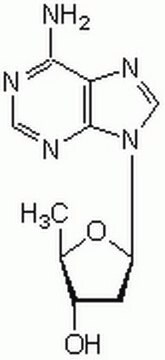
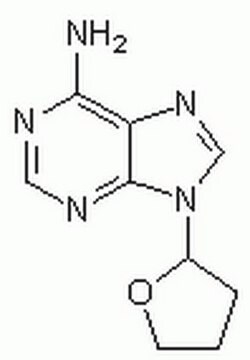
2′,5′-Dideoxyadenosine
≥95% (HPLC), solid
Synonym(s):
2ʹ,5ʹ-dd-Ado, NSC 95943
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H13N5O2
CAS Number:
Molecular Weight:
235.24
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥95% (HPLC)
form
solid
color
white
solubility
DMSO: soluble
storage temp.
−20°C
SMILES string
C[C@H]1O[C@H](C[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H13N5O2/c1-5-6(16)2-7(17-5)15-4-14-8-9(11)12-3-13-10(8)15/h3-7,16H,2H2,1H3,(H2,11,12,13)/t5-,6+,7-/m1/s1
InChI key
FFHPXOJTVQDVMO-DSYKOEDSSA-N
Gene Information
rat ... Adcy2(81636)
Application
2′,5′-Dideoxyadenosine has been used to elucidate the mechanism of diligustilide (DLG). It has also been used to inhibit adenylate cyclase (AC).
Biochem/physiol Actions
Cell-permeable adenylyl cyclase inhibitor. IC50 = 2.7 μM in detergent-dispersed rat brain preparations.
Features and Benefits
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Adenylyl cyclases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Reconstitution
Store at −20 °C after reconstitution.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rula Azzam et al.
AIDS research and human retroviruses, 22(7), 619-629 (2006-07-13)
HIV-1 infection of cells of macrophage lineage impairs a number of effector functions performed by these cells, including phagocytosis of opsonized pathogens. In this study we investigate the effects of HIV-1 on the mechanism of complement (C')-mediated phagocytosis by human
Gastroprotective effect of diligustilide isolated from roots of Ligusticum porteri coulter & rose (Apiaceae) on ethanol-induced lesions in rats
Velazquez-Moyado J, et al.
Journal of Ethnopharmacology, 174, 403-409 (2015)
Yukihisa Matsumoto et al.
PloS one, 8(7), e68538-e68538 (2013-07-31)
Many insects exhibit excellent capability of visual learning, but the molecular and neural mechanisms are poorly understood. This is in contrast to accumulation of information on molecular and neural mechanisms of olfactory learning in insects. In olfactory learning in insects
Fen Hu et al.
Molecular medicine reports, 24(4) (2021-07-31)
Inflammation and oxidative stress have indispensable roles in the development of acute lung injury (ALI). MicroRNA (miRNA/miR)‑351‑5p was initially identified as a myogenesis‑associated miRNA; however, its role in lipopolysaccharide (LPS)‑induced ALI remains unclear. The aim of the present study was
Hyun-Seuk Moon et al.
Journal of cellular physiology, 214(2), 283-294 (2007-07-27)
We previously reported that PEGylated conjugated linoleic acid (PCLA) as a pro-drug treatment of cultures of 3T3-L1 cells containing differentiated adipocytes caused de-differentiation by downregulation of PPARgamma2-induced adipogenesis, and cell apoptosis induced by PCLA was lower than that induced by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service