C3735
Cytochrome P450 human
1A1 Isozyme Microsomes, with P450 Reductase, recombinant, expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12161501
NACRES:
NA.47
Recommended Products
biological source
human
Quality Level
recombinant
expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
form
solution
specific activity
≥4 units/pmol enzyme
mol wt
45-60 kDa
packaging
vial of 0.5 nmol
technique(s)
activity assay: suitable
solubility
water: soluble
suitability
suitable for molecular biology
UniProt accession no.
application(s)
cell analysis
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... CYP1A1(1543)
Looking for similar products? Visit Product Comparison Guide
General description
Research area: Immuno and CKS
Cytochrome P450 (CYP) enzymes are a family of enzymes that are encoded by the P450 genes. These enzymes are membrane bound hemoproteins. that are mainly found in the liver’s endoplasmic reticulum.
Cytochrome P450 (CYP) enzymes are a family of enzymes that are encoded by the P450 genes. These enzymes are membrane bound hemoproteins. that are mainly found in the liver’s endoplasmic reticulum.
Biochem/physiol Actions
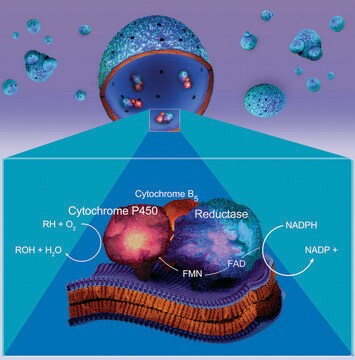
Cytochrome P450 is a heterogeneous family of isozymes whose primary function is to oxidize small molecules, both as a function of intermediary metabolism (e.g., fatty acids) and to detoxify exogenous compounds (drugs or toxins). Some isoforms have narrow substrate specificity, while others are promiscuous. The CYP1A1 isoform catalyzes 7-deethylation of ethoxyresorufin. Cytochrome P450 (CYP) plays an important role in detoxifying xenobiotics, cellular metabolism and homeostasis. One of the main mechanisms of drug-drug interactions is the induction or inhibition of these enzymes. CYP enzymes are transcriptionally activated by a variety of xenobiotics and by endogenous substrates via receptor-dependent pathways. Inhibition of these enzymes is a major factor in metabolism-based drug-drug interactions, and many chemotherapeutic medications can cause drug interactions by either inhibiting or inducing the cytochrome p450 enzyme system.
Physical form
Solution in 100 mM potassium phosphate buffer, pH 7.4.
Preparation Note
Microsomes containing human CYP1A1 and recombinant human NADPH-P450 reductase.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tomas Erban et al.
Biomedical chromatography : BMC, 26(9), 1062-1065 (2011-11-29)
A 96-well microplate-based HPLC endpoint assay is described for the determination of NADPH-cytochrome P450 reductase (CPR) activity. Novel sampling of NADPH into microplates was optimized. Separation was performed on a Zorbax Eclipse XDB-C₁₈ analytical 4.6 × 150 mm, 5 µm column. To validate the
David Stucki et al.
Archives of biochemistry and biophysics, 687, 108383-108383 (2020-04-27)
Intracellular carbon monoxide (CO) is a gaseous signaling molecule and is generated enzymatically by heme oxygenases upon degradation of heme to billiverdin. Target structures for intracellular produced CO are heme proteins including cytochrome c oxidase of the respiratory chain, cytochrome
D P Bofinger et al.
Toxicological sciences : an official journal of the Society of Toxicology, 62(2), 299-314 (2001-07-14)
Endometriosis is a debilitating disease estimated to affect 10% of reproductive-age women and characterized by the growth of endometrial tissue outside of the uterus. The present study characterizes a human endometrial explant culture model for studying the direct effects of
Moritz Walter et al.
Toxicology in vitro : an international journal published in association with BIBRA, 59, 215-220 (2019-04-21)
Next to its well-studied toxicity, carbon monoxide (CO) is recognized as a signalling molecule in various cellular processes. Thus, CO-releasing molecules (CORMs) are of considerable interest for basic research and drug development. Aim of the present study was to investigate
Xiangrong Zhang et al.
PloS one, 9(4), e94962-e94962 (2014-04-17)
The present study characterized in vitro metabolites of 20(R)-25-methoxyl-dammarane-3β, 12β, 20-triol (20(R)-25-OCH3-PPD) in mouse, rat, dog, monkey and human liver microsomes. 20(R)-25-OCH3-PPD was incubated with liver microsomes in the presence of NADPH. The reaction mixtures and the metabolites were identified
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service