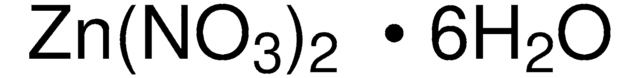All Photos(3)
About This Item
Linear Formula:
Zn(NO3)2 · 6H2O
CAS Number:
Molecular Weight:
297.49
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.21
Assay:
98%
grade:
reagent grade
form:
flakes
powder or crystals
powder or crystals
solubility:
water: soluble(lit.)
Recommended Products
grade
reagent grade
Quality Level
Assay
98%
form
flakes
powder or crystals
solubility
water: soluble(lit.)
SMILES string
O.O.O.O.O.O.[Zn++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/2NO3.6H2O.Zn/c2*2-1(3)4;;;;;;;/h;;6*1H2;/q2*-1;;;;;;;+2
InChI key
JGPSMWXKRPZZRG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Used to synthesize a new microporous, organically templated zinc phosphate. Such compounds have potential applications as catalysts or in other applications involving microporosity.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xuemin Qian et al.
Nanoscale research letters, 3(8), 303-307 (2008-01-01)
ZnO nanorod arrays are prepared on a silicon wafer through a multi-step hydrothermal process. The aspect ratios and densities of the ZnO nanorod arrays are controlled by adjusting the reaction times and concentrations of solution. The investigation of field emission
Milna GWA.
Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties, 4153-4153 (2015)
Structural and DC electrical investigations of ZnO thin films prepared by spray pyrolysis technique.
Riad AS, et al.
Physica B: Condensed Matter (Amsterdam, Netherlands), 296(4), 319-325 (2001)
Robert J Friederichs et al.
Journal of the Royal Society, Interface, 12(108), 20150190-20150190 (2015-06-05)
Experimental chemistry and atomic modelling studies were performed here to investigate a novel ionic co-substitution in hydroxyapatite (HA). Zinc, silicate co-substituted HA (ZnSiHA) remained phase pure after heating to 1100 °C with Zn and Si amounts of 0.6 wt% and
Harmon, S.B. Sevov, S.C.
Chemistry of Materials, 10, 3020-3020 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service