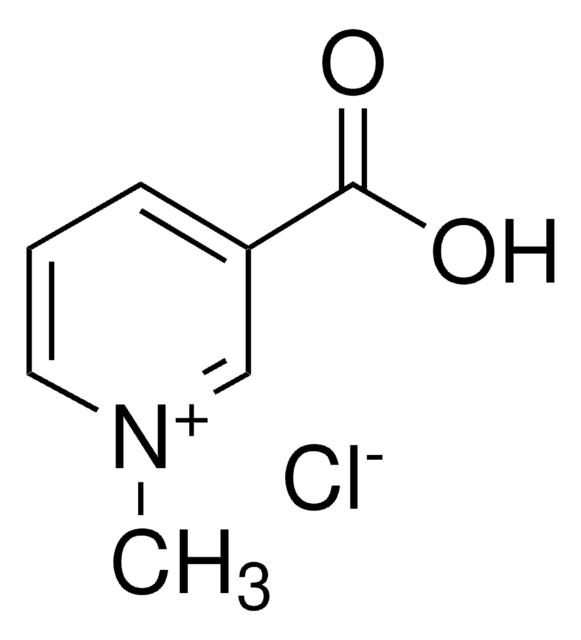
M4627
1-Methylnicotinamide chloride
analytical standard
Synonym(s):
N-Methylnicotinic acid amide chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C7H9N2OCl
CAS Number:
Molecular Weight:
172.61
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥97.5%
form
powder
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
color
white
application(s)
forensics and toxicology
veterinary
vitamins, nutraceuticals, and natural products
format
neat
SMILES string
[Cl-].C[n+]1cccc(c1)C(N)=O
InChI
1S/C7H8N2O.ClH/c1-9-4-2-3-6(5-9)7(8)10;/h2-5H,1H3,(H-,8,10);1H
InChI key
BWVDQVQUNNBTLK-UHFFFAOYSA-N
General description
1-Methylnicotinamide (MNA) is a primary metabolite of nicotinamide produced mainly by nicotinamide N-methyltransferase (NNMT) and exhibits antithrombotic and anti-inflammatory effects.
Application
1-Methylnicotinamide chloride may be used as a standard in the quantification of 1-methylnicotinamide in urine samples using ion pairing reverse-phase high performance liquid chromatography (RP-HPLC).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Biochem/physiol Actions
Urinary excretion product of niacin metabolism
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stefan Chłopicki et al.
Pharmacological reports : PR, 64(2), 369-376 (2012-06-05)
Methylnicotinamide (MNA) displays vasoprotective activity, however, the regulation of the activity of nicotinamide-N-methyltransferase (NNMT), is largely unknown. We analyze a possible involvement of IL-6 in the activation of NNMT-MNA pathway during an endurance exercise. FVB, C57Bl/6J IL6(+/+) and C57Bl/6J IL-6(-/-)
Magdalena Sternak et al.
Pharmacological reports : PR, 62(3), 483-493 (2010-07-16)
Nicotinamide N-methyltransferase (NNMT), which converts nicotinamide (NA) to 1-methylnicotinamide (MNA), is up-regulated in the cirrhotic liver. Because MNA displays PGI(2)-dependent anti-inflammatory effects, the up-regulation of NNMT may play a regulatory role in liver inflammation. In the present work, we analyzed
Misha Patel et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 921-922, 87-95 (2013-02-26)
Nicotinamide N-methyltransferase (NNMT, E.C. 2.1.1.1) N-methylates nicotinamide to produce 1-methylnicotinamide. Enhanced NNMT activity is a feature of many types of cancer, and has been linked to processes such as tumour metastasis, resistance to radiotherapy and tumour drug resistance. As such
Tamara Kuchmerovska et al.
Neurochemistry international, 56(2), 221-228 (2009-10-20)
The present study has been designed to establish the potential benefits from 1-methylnicotinamide (MNA) treatment on brain disorders associated with type 1 diabetes. All experiments were carried out after 6 weeks of streptozotocin-induced diabetes (60 mg/kg of body weight, i.p.)
Wu-Ping Sun et al.
Hypertension research : official journal of the Japanese Society of Hypertension, 35(2), 180-185 (2011-09-16)
Nicotinamide and catecholamines are both degraded by S-adenosylmethionine-dependent methylation. Whether excess nicotinamide affects the degradation of catecholamines is unknown. The aim of this study was to investigate the effect of nicotinamide on the methylation status of the body and methylation-mediated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service