52341
Salicylic acid
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonym(s):
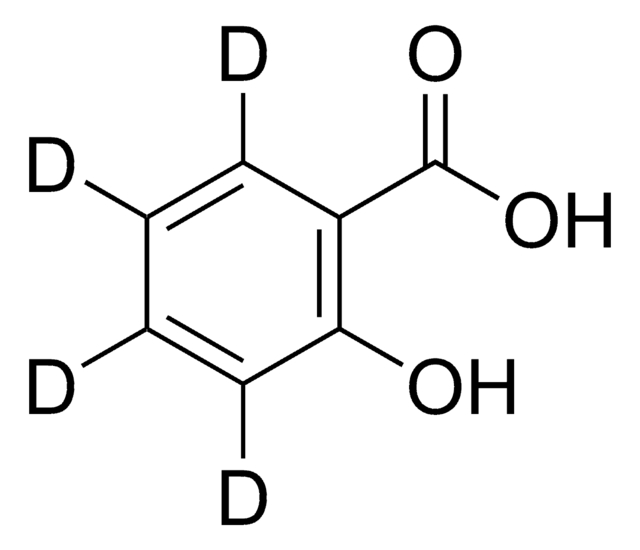
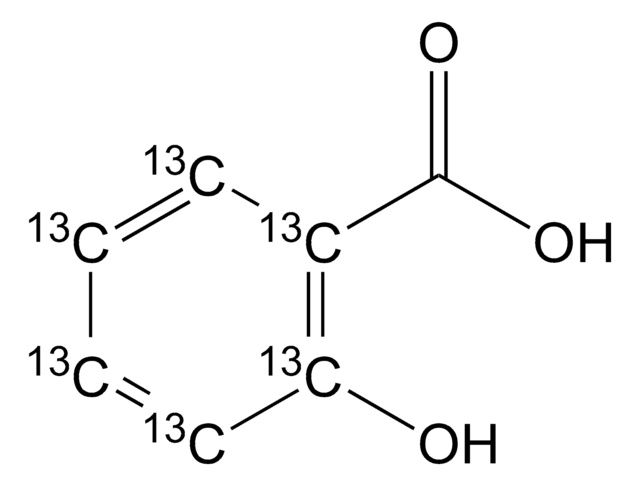
2-Hydroxybenzoic acid
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
vapor density
4.8 (vs air)
vapor pressure
1 mmHg ( 114 °C)
product line
TraceCERT®
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
211 °C (lit.)
mp
158-161 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
pharmaceutical
format
neat
SMILES string
OC(=O)c1ccccc1O
InChI
1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10)
InChI key
YGSDEFSMJLZEOE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Application
Packaging
Other Notes
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
314.6 °F - closed cup
Flash Point(C)
157 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Acetylsalicylic acid, analytical standard; Salicylic acid, BioXtra, ≥99.0%
Separation of Salicylic acid, meets analytical specification of Ph. Eur., BP, USP, 99.5-100.5% (calc. to the dried substance); Acetylsalicylic acid, purum, ≥99.0% (HPLC)
Separation of 4-Hydroxybenzoic acid; Acetylsalicylic acid; Benzoic acid; Salicylic acid; Ethyl 4-hydroxybenzoate
HPLC Analysis of Benzoic Acid Derivatives on Ascentis® C18
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service