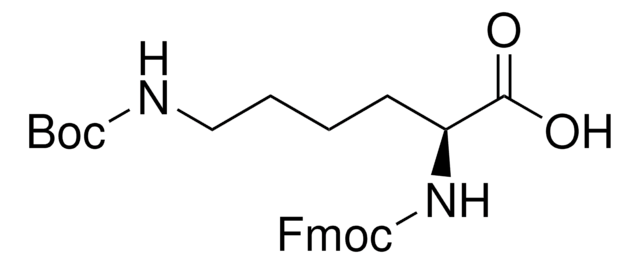
8.52000
Fmoc-Thr(tBu)-OH
Novabiochem®
Synonym(s):
Fmoc-Thr(tBu)-OH, N-α-Fmoc-O-t.-butyl-L-threonine
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
Assay
≥98% (TLC)
≥98.0% (acidimetric)
≥99.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
mp
125-135 °C
application(s)
peptide synthesis
functional group
hydroxyl
storage temp.
2-30°C
InChI
1S/C23H27NO5/c1-14(29-23(2,3)4)20(21(25)26)24-22(27)28-13-19-17-11-7-5-9-15(17)16-10-6-8-12-18(16)19/h5-12,14,19-20H,13H2,1-4H3,(H,24,27)(H,25,26)/p-1/t14-,20+/m1/s1
InChI key
LZOLWEQBVPVDPR-VLIAUNLRSA-M
General description
Standard building block for introduction of threonine amino-acid residues by Fmoc SPPS
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Application
- Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group: This study demonstrates the effective use of Fmoc-Thr(tBu)-OH in the synthesis of N-methyl amino acids, which are crucial in developing peptidomimetic drugs (Román et al., 2023).
- Controlled Morphological Changes in Self-Assembled Structures Formed by Fmoc Variants of Threonine and Serine: Explores how Fmoc-Thr(tBu)-OH influences the self-assembly and structural morphology of peptidic materials, relevant in material science (Kshtriya et al., 2021).
- Solid-phase Synthesis of C-terminus Cysteine Peptide Acids: This article includes the use of Fmoc-Thr(tBu)-OH in the synthesis of peptides containing cysteine, which are significant in both drug design and biochemical studies (Mthembu et al., 2022).
- Polymer-Supported Stereoselective Synthesis of Benzoxazinothiadiazepinone 6,6-dioxides: Discusses the use of Fmoc-Thr(tBu)-OH in the stereoselective synthesis of novel organic compounds with potential applications in medicinal chemistry (Králová et al., 2017).
Linkage
Analysis Note
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr(tBu)-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (GC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (25 mmole in 50 ml DMF): clearly soluble
Assay (acidimetric): ≥ 98.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service