999995P
Avanti
Edelfosine
Avanti Research™ - A Croda Brand 999995P, powder
Synonym(s):
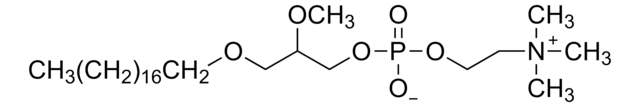
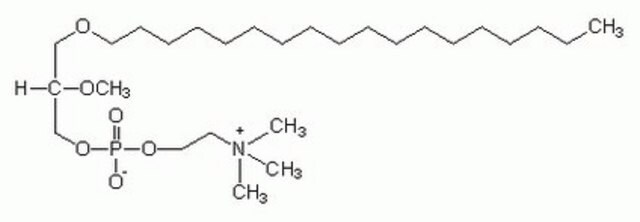
1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine
About This Item
Recommended Products
form
powder
packaging
pkg of 1 × 5 mg (999995P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 999995P
lipid type
cardiolipins
phospholipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
[O-]P(OCC[N+](C)(C)C)(OC[C@]([H])(OC)COCCCCCCCCCCCCCCCCCC)=O
InChI
1S/C10H24NO5P/c1-6-10(14-5)9-16-17(12,13)15-8-7-11(2,3)4/h10H,6-9H2,1-5H3/t10-/m0/s1
InChI key
GVMCXWJIRSIWFJ-JTQLQIEISA-N
Application
- as a non-hydrolysable LysoPC (phospholipid) analog for analyzing its ability to block sexual commitment in Plasmodium falciparum
- in multilamellar vesicle preparation, to study its effect on model membranes
- in the selection and screening of mutagenized cells, having the ability to inhibit the transport of alkylphosphocholine drugs across the plasma membrane
Biochem/physiol Actions
Packaging
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service