790528P
Avanti
18:1 DGS-NTA
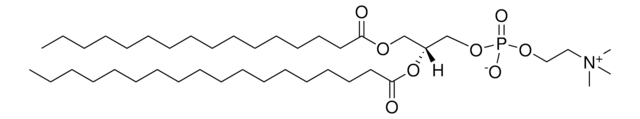
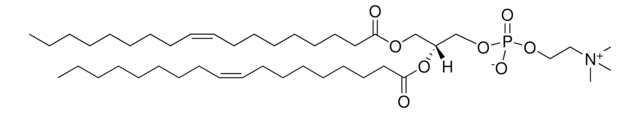
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (ammonium salt), powder
Synonym(s):
1,2-di-(9Z-octadecenoyl)-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (ammonium salt); DOGS NTA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C53H100N5O13
CAS Number:
Molecular Weight:
1015.39
MDL number:
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 5 mg (790528P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 790528P
shipped in
dry ice
storage temp.
−20°C
General description
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (18:1 DGS-NTA) is amphiphile. DGS-NTA is a nitrilotriacetic acid -functionalized amphiphile devoid of nickel ion.
Application
18:1 DGS-NTA 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (ammonium salt) has been used:
- for generation of amphiphile monolayer
- in NTA-liposome preparation for procoagulant binding studies and to study its effect on factor FXII autoactivation
- as a liposome component for lipid bilayer preparation for surface plasmon resonance studies
Biochem/physiol Actions
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (18:1 DGS-NTA or DOGS-NTA) cannot bind to histidine due to lack of nickel ion. It is capable of self-assembly to form a stable monolayer. DOGS-NTA ammonium salt is useful in bicelle preparation.
Packaging
5 mL Clear Glass Sealed Ampule (790528P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Louis Gioia et al.
Science immunology, 4(38) (2019-09-01)
The class II region of the major histocompatibility complex (MHC) locus is the main contributor to the genetic susceptibility to type 1 diabetes (T1D). The loss of an aspartic acid at position 57 of diabetogenic HLA-DQβ chains supports this association;
N Bischler et al.
Biophysical journal, 74(3), 1522-1532 (1998-03-25)
Nickel-chelating lipid monolayers were used to generate two-dimensional crystals from yeast RNA polymerase I that was histidine-tagged on one of its subunits. The interaction of the enzyme with the spread lipid layers was found to be imidazole dependent, and the
E Barklis et al.
The Journal of biological chemistry, 273(13), 7177-7180 (1998-04-29)
In an in vitro system that mimics the assembly of immature human immunodeficiency virus (HIV) particles, ordered arrays of HIV-1 capsid (CA) proteins encoded by the viral gag gene have been obtained by incubation of histidine-tagged capsid proteins (His-HIVCA) beneath
C Dietrich et al.
Biochemistry, 35(4), 1100-1105 (1996-01-30)
The coupling of a DNA-binding protein to self-organized lipid monolayers is examined at the air-water interface by means of film balance techniques and epifluorescence microscopy. We used two recombinant species of the heat shock factor HSF24 which differ only in
C Vénien-Bryan et al.
Biophysical journal, 74(5), 2649-2657 (1998-05-20)
We present here some sensitive optical and mechanical experiments for monitoring the process of formation and growth of two-dimensional (2D) crystals of proteins on a lipid monolayer at an air-water interface. The adsorption of proteins on the lipid monolayer was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![18:1 DGS-NTA(Co) 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (Cobalt salt), powder](/deepweb/assets/sigmaaldrich/product/structures/203/482/b877a7a9-e8a9-4cec-99cf-b256a26d690c/640/b877a7a9-e8a9-4cec-99cf-b256a26d690c.png)