W506907
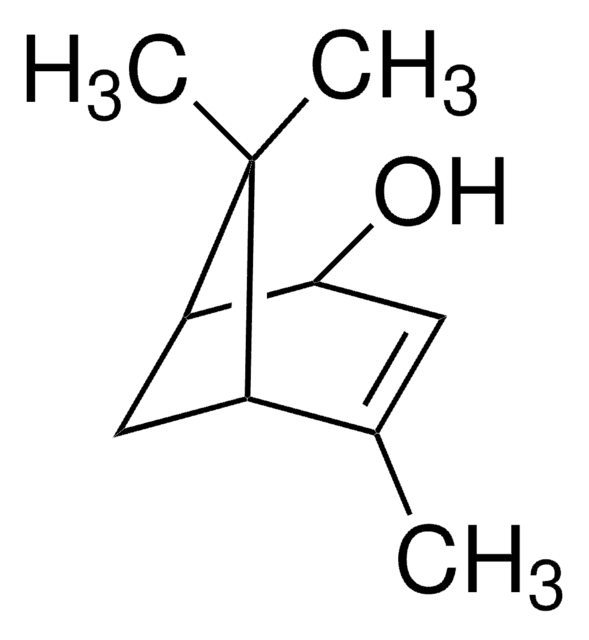
(1S)-(−)-Verbenone
≥93%
Synonym(s):
(1S,5S)-2-Pinen-4-one, (1S,5S)-4,6,6-Trimethylbicyclo[3.1.1]hept-3-en-2-one
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
Assay
≥93%
optical activity
[α]20/D −140°, c = 10 in ethanol
refractive index
n20/D 1.496 (lit.)
bp
227-228 °C (lit.)
density
0.975 g/mL at 20 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
camphoraceous; minty; spicy
SMILES string
CC1=CC(=O)[C@H]2C[C@@H]1C2(C)C
InChI
1S/C10H14O/c1-6-4-9(11)8-5-7(6)10(8,2)3/h4,7-8H,5H2,1-3H3/t7-,8+/m0/s1
InChI key
DCSCXTJOXBUFGB-JGVFFNPUSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Anti-Inflammatory and Antinociceptive Activities of the Essential Oil of Tagetes parryi A. Gray (Asteraceae) and Verbenone.: This article reports on the anti-inflammatory and pain-relief effects of (1S)-(−)-Verbenone as part of the essential oil of Tagetes parryi, suggesting potential therapeutic applications for pain management and inflammation (González-Velasco et al., 2022).
Disclaimer
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
185.0 °F - closed cup
Flash Point(C)
85 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
suitable for GCsuitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)