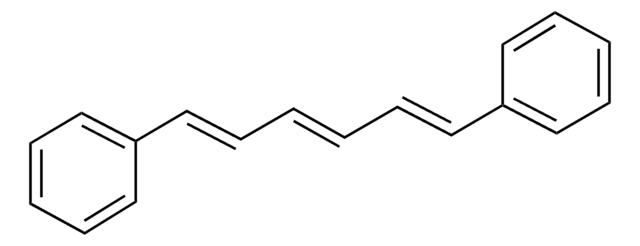
D206008
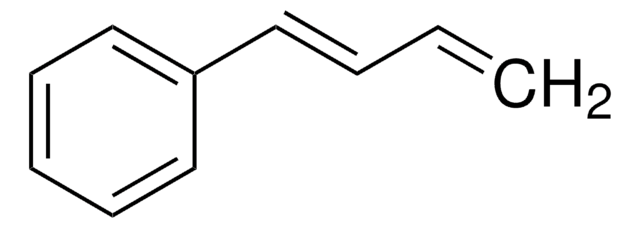
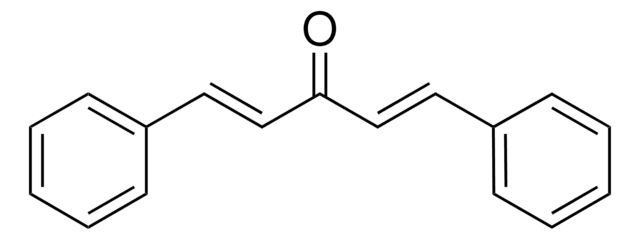
trans,trans-1,4-Diphenyl-1,3-butadiene
98%
Synonym(s):
β,β′-Bistyryl, DPB
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH=CHCH=CHC6H5
CAS Number:
Molecular Weight:
206.28
Beilstein:
1905939
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
350 °C (lit.)
mp
150-152 °C (lit.)
SMILES string
c1ccc(cc1)\C=C\C=C\c2ccccc2
InChI
1S/C16H14/c1-3-9-15(10-4-1)13-7-8-14-16-11-5-2-6-12-16/h1-14H/b13-7+,14-8+
InChI key
JFLKFZNIIQFQBS-FNCQTZNRSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
trans,trans-1,4-Diphenyl-1,3-butadiene can be used as a reactant to synthesize:
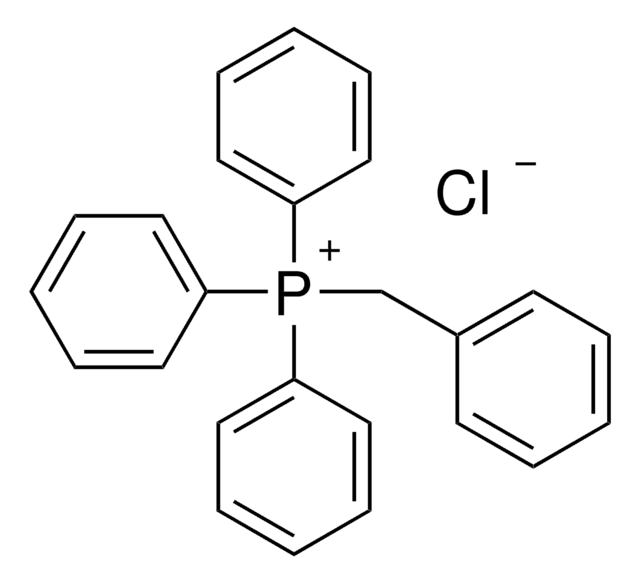
It can also be used as a ligand to prepare silver(I) coordination polymers by reacting with silver(I) salts.
- 2,5-diphenylthiophene by oxidation reaction with potassium sulfide and DMSO.
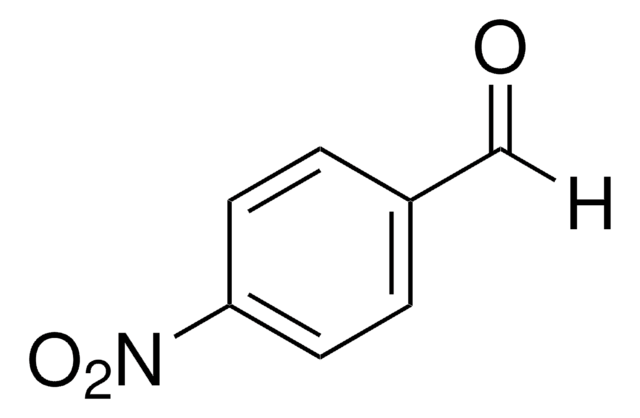
- 2-[(3E)-4-Phenyl-2-(phenylmethyl)-3-buten-1-yl]furan via nickel catalyzed hydrobenzylation reaction with furfural in the presence of N2H4.
It can also be used as a ligand to prepare silver(I) coordination polymers by reacting with silver(I) salts.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
David P Burgner et al.
PloS one, 10(5), e0125342-e0125342 (2015-05-06)
Pathogen-specific and overall infection burden may contribute to atherosclerosis and cardiovascular disease (CVD), but the effect of infection severity and timing is unknown. We investigated whether childhood infection-related hospitalisation (IRH, a marker of severity) was associated with subsequent adult CVD
Liang Chen et al.
Organic letters, 20(23), 7392-7395 (2018-11-22)
A novel, atom economical, and transition-metal-free strategy for the synthesis of thiophenes from substituted buta-1-enes with potassium sulfide has been presented. The reaction achieves double C-S bond formations via cleavage of multiple C-H bonds and provides an efficient approach to
Mustafa Toprakçí et al.
Bioorganic & medicinal chemistry letters, 15(20), 4438-4446 (2005-09-03)
Monoamine oxidase (EC1.4.3.4; MAO) is a mitochondrial outer membrane flavoenzyme that catalyzes the oxidation of biogenic amines. It has two distinct isozymic forms designated MAO-A and MAO-B, each displaying different substrate and inhibitor specificities. They are the well-known targets for
Vikram Agarwal et al.
eLife, 4 (2015-08-13)
MicroRNA targets are often recognized through pairing between the miRNA seed region and complementary sites within target mRNAs, but not all of these canonical sites are equally effective, and both computational and in vivo UV-crosslinking approaches suggest that many mRNAs
Samim Sardar et al.
Scientific reports, 5, 17313-17313 (2015-11-28)
Energy harvesting from solar light employing nanostructured materials offer an economic way to resolve energy and environmental issues. We have developed an efficient light harvesting heterostructure based on poly(diphenylbutadiyne) (PDPB) nanofibers and ZnO nanoparticles (NPs) via a solution phase synthetic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service