D18609
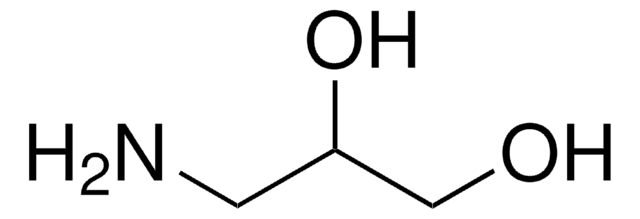
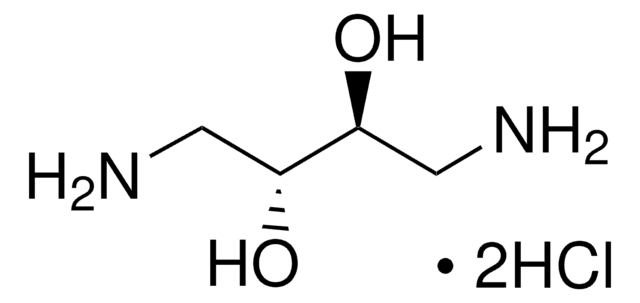
1,3-Diamino-2-propanol
95%
Synonym(s):
2-Hydroxy-1,3-propanediamine, 1,3-Diamino-2-hydroxypropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CH(OH)CH2NH2
CAS Number:
Molecular Weight:
90.12
Beilstein:
741859
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
40-44 °C (lit.)
SMILES string
NCC(O)CN
InChI
1S/C3H10N2O/c4-1-3(6)2-5/h3,6H,1-2,4-5H2
InChI key
UYBWIEGTWASWSR-UHFFFAOYSA-N
Gene Information
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- 1,3-Diamino-2-propanol is a versatile bidentate diamine ligand which is used in the synthesis of a variety of organometallic compounds.
- It is a precursor to synthesize the fluorogenic dsDNA binder, N1,N3-bis(4-amidinophenyl)propane-1,3-diamine (BAPPA).
- It can also be used as a branching unit in the synthesis of peptide dendrimers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient Phosphodiester Binding and Cleavage by a ZnII Complex Combining Hydrogen?Bonding Interactions and Double Lewis Acid Activation.
Feng G, et al.
Angewandte Chemie (International Edition in English), 45(42), 7056-7059 (2006)
Synthesis and esterolytic activity of catalytic peptide dendrimers.
Lagnoux D, et al.
Chemistry?A European Journal , 10(5), 1215-1226 (2004)
Syntheses, structures, and electrochemical properties of inclusion compounds of cucurbit [8] uril with cobalt (III) and nickel (II) complexes.
Mitkina T V, et al.
Inorganic Chemistry, 47(15), 6748-6755 (2008)
Structure? activity relationships for cytotoxic ruthenium (II) arene complexes containing N, N-, N, O-, and O, O-chelating ligands.
Habtemariam A, et al.
Journal of Medicinal Chemistry, 49(23), 6858-6868 (2006)
Bis-4-aminobenzamidines: versatile, fluorogenic A/T-selective dsDNA binders.
Vazquez O, et al.
Organic Letters, 12(2), 216-219 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service